How does the Brine Edge X Superlight lacrosse head perform for different positions. What are the key features and benefits of this versatile lacrosse head. Is the Edge X Superlight a good value for players at various skill levels.
Overview of the Brine Edge X Superlight Lacrosse Head
The Brine Edge X Superlight is a versatile lacrosse head designed to offer high performance for players across multiple positions. Priced at $84.99, this updated version of Brine’s classic Edge head maintains the defensive prowess of its predecessors while incorporating new features to enhance its all-around capabilities.
Key specifications of the Edge X Superlight include:
- Universal head specs suitable for offense and defense
- “Superlight” construction for reduced weight
- Retail price of $84.99
- Available in classic Edge styling
Design and Appearance: Blending Tradition with Innovation
The Edge X Superlight stays true to the iconic Edge design while incorporating subtle updates. How does it compare visually to other modern lacrosse heads? The reviewer notes that Brine has wisely chosen not to drastically alter the classic Edge aesthetic, avoiding unnecessary visual gimmicks that often clutter the current lacrosse gear market.

Notable design elements include:
- Classic Edge head shape and profile
- Minimal visual updates from previous models
- Clean, timeless appearance
Stringing Capabilities and Pocket Customization
Stringing options are a crucial factor for many players when selecting a lacrosse head. Does the Edge X Superlight offer sufficient versatility in this area? According to the review, the head provides ample stringing holes and customization potential.
Key stringing features:
- Multiple sidewall holes for diverse stringing patterns
- Large scoop slots to accommodate thick top strings
- Ability to create various pocket styles (though natural channel may be limited)
While the reviewer notes that the wide head design may limit natural channeling, skilled stringers should be able to achieve their desired pocket configuration with some creativity.
Considerations for Different Pocket Types
The Edge X Superlight’s versatile design allows for various pocket styles. Which types of pockets might work best with this head? Consider the following options:

- Low pocket: Ideal for quick release and ball control
- Mid pocket: Balanced option for all-around play
- High pocket: Suited for defenders looking for maximum hold
Players should experiment with different stringing patterns to find the optimal setup for their playing style and position.
Stiffness and Flexibility: Balancing Defensive Strength with Offensive Finesse
As a head marketed for both offensive and defensive use, how does the Edge X Superlight balance stiffness and flexibility? The reviewer conducted hands-on testing to evaluate its performance in various situations.
Findings on stiffness and flexibility:
- Surprisingly stiff for a “Superlight” construction
- Effective for defensive poke checks
- Slight give compared to standard Edge X, but still robust
- Flexible sidewalls beneficial for face-offs
This balance of stiffness and flexibility makes the Edge X Superlight suitable for multi-position players or those transitioning between roles on the field.
Durability and Construction: Built to Last
Durability is a key concern for players at all levels. How does the Edge X Superlight hold up to the rigors of regular play? The reviewer highlights several design elements that contribute to the head’s longevity:

- Thick scoop and sidewall junctions for reinforcement
- Extended neck to support the head and prevent breakage
- “Superlight” materials that maintain strength while reducing weight
These features suggest that the Edge X Superlight should withstand intense gameplay and practice sessions, offering good value for its price point.
Maintenance Tips for Longevity
To maximize the lifespan of your Edge X Superlight, consider the following maintenance practices:
- Regularly inspect for cracks or weak points
- Clean the head after use to prevent dirt buildup
- Store in a cool, dry place away from direct sunlight
- Avoid leaving in hot cars or extreme temperatures
Value Proposition: Affordable Excellence for Various Skill Levels
At $84.99, the Edge X Superlight sits in the mid-range of lacrosse head pricing. Is it a good value for players? The reviewer assigns a perfect 10/10 for value, citing several factors:
- Versatility across multiple positions
- Lightweight design beneficial for younger or developing players
- Durable construction for long-term use
- Reasonable price point compared to high-end specialty heads
While it is $15 more expensive than the standard Edge X, the reviewer suggests that the weight reduction justifies the price difference, especially for younger players or those looking to improve their stick skills.

Player Profiles: Who Benefits Most from the Edge X Superlight?
The Edge X Superlight’s versatile design makes it suitable for a wide range of players. Which positions and skill levels might find this head most beneficial?
Ideal player profiles for the Edge X Superlight:
- Intermediate-level players in any position
- Youth players looking for a lightweight, all-around head
- Defensive midfielders requiring both offensive and defensive capabilities
- Crease attackmen valuing quick release and maneuverability
- Face-off specialists appreciating the flexible sidewalls
The reviewer notes that even lacrosse legend Casey Powell cites the Edge as his all-time favorite head, further emphasizing its versatility beyond purely defensive applications.
Skill Development Opportunities
How can players leverage the Edge X Superlight to improve their game? Consider focusing on these skills:
- Ground ball pickups: Utilize the reinforced scoop for clean scoops
- Defensive checks: Practice poke and slap checks with the stiff frame
- Quick stick skills: Take advantage of the lightweight design for faster passes and shots
- Face-off techniques: Experiment with the flexible sidewalls for improved clamp strength
Comparative Analysis: Edge X Superlight vs. Competitors
To fully evaluate the Edge X Superlight, it’s important to consider how it stacks up against similar lacrosse heads in the market. How does it compare to other versatile or position-specific options?

Potential comparisons to consider:
- Edge X Superlight vs. Standard Edge X: Weight difference and performance trade-offs
- Edge X Superlight vs. Offensive-focused heads: Versatility and shooting accuracy
- Edge X Superlight vs. Other all-position heads: Price, durability, and overall performance
While the review doesn’t provide direct comparisons, players should research and potentially test multiple options to find the best fit for their playing style and needs.
Key Differentiators
What sets the Edge X Superlight apart from its competitors? Consider these unique selling points:
- Legacy of the Edge series combined with modern lightweight materials
- Balance of defensive stiffness and offensive maneuverability
- Attractive price point for a versatile, high-performance head
- Endorsement from top players like Casey Powell
Overall Performance and Recommendations
The reviewer assigns the Brine Edge X Superlight an overall score of 8.2/10, indicating strong performance across various criteria. What are the key takeaways for potential buyers?

Summary of strengths:
- Versatile design suitable for multiple positions
- Lightweight construction without sacrificing durability
- Classic aesthetics with modern performance
- Excellent value for the price
- Ample stringing options for customization
Areas for consideration:
- Slightly less stiff than standard Edge X (may affect defensive specialists)
- Limited natural channel due to wide design (may require skilled stringing)
The Edge X Superlight appears to be an excellent choice for players seeking a versatile, lightweight head that can adapt to various positions and play styles. Its combination of performance, durability, and value make it a strong contender in the mid-range lacrosse head market.
Final Thoughts
Is the Brine Edge X Superlight right for you? Consider these factors when making your decision:
- Your primary playing position and potential for multi-position play
- Importance of weight reduction in your gameplay
- Budget constraints and value expectations
- Preference for classic design aesthetics
- Need for versatility in stringing options
By carefully evaluating these aspects in relation to your personal needs and playing style, you can determine if the Edge X Superlight is the ideal lacrosse head for your game.

Gear Review: Edge X Superlight by Brine Lacrosse
[rwp-review id=”1″]
Company: Brine Lacrosse / Product: Edge X Superlight Head / Price: $84.99
The Edge is famous for being an elite defensive head, and while this incarnation is no different, it does provide more versatility due to its universal specs and “Superlight” construction. As an offensive player, I was curious to see how I’d like this head, and was overall impressed with how it performed.
The Edge X Superlight.
Appearance… +10.0
This head is a classic, and Brine has done very little to update it visually…but that is a good thing! Why mess with a classic, especially in a market diluted by visual gimmicks.
Nice touch on a classic-looking head.
Stringing… +7.5
My buddy strung this up for me with my preferred low pocket, and I tweaked it a bit once I’d thrown with it awhile. There wasn’t much of a channel in this head, partially be design and partially due to the wideness of the head. I imagine one could string this with a greater channel if they wanted to, though. There were plenty of sidewall holes to use, and the scoop of the head has large slots for the top string, just as previous models of the Edge have had. This allowed us to use a super thick top string that will last longer.
I imagine one could string this with a greater channel if they wanted to, though. There were plenty of sidewall holes to use, and the scoop of the head has large slots for the top string, just as previous models of the Edge have had. This allowed us to use a super thick top string that will last longer.
Plenty of side wall holes, plus large slots for the top string are all great features of the Edge.
Stiffness… +7.5
As a defensive head, you would want this to be very stiff. However, as a Superlight head, I was worried it might not be. To test the head, I gave some hard poke checks to various surfaces, and I was surprised to find that while this head might give a little more than the “normal” Edge X, it really bit well on checks. Interestingly, I talked to Team TLN face-off man Heeyoung Leem while testing this, and he said the all Superlight heads are great for facing off because they flex so well at the sidewalls.
Wall Ball Wednesday.
Durability… +7.5
Something I really like about this head is how thick the scoop and corners of the head are. By corners, I mean the spot where the scoop meets the sidewall. This not only makes ground balls easier to pick up, but it also reinforces the head to help counteract the flexibility stemming from how light it is. The Edge also has a long neck that will help support it and keep from breaking.
By corners, I mean the spot where the scoop meets the sidewall. This not only makes ground balls easier to pick up, but it also reinforces the head to help counteract the flexibility stemming from how light it is. The Edge also has a long neck that will help support it and keep from breaking.
Plenty of side wall holes, plus large slots for the top string are all great features of the Edge.
Value… +10.0
At $85, this isn’t the most expensive head out there, and it provides a lot of good aspects. I can see intermediate-level players of any position using this head because it’s so light. It is $15 more than the non-Superlight Edge X, but I think its low weight is a plus, especially for younger players.
Overall… 8.2/10
In a recent Hangout on The Lacrosse Network, lacrosse legend Casey Powell said that his all time favorite head is the Edge. If this doesn’t tell you that the head isn’t defensive-specific, I don’t know what does. Wider AND lighter than most, this head would be great for younger players, crease attackmen, defensive middies, and CP22.
Brine Phantom X Soccer Ball
Product Description
Brine Phantom X Soccer Ball
A match level soccer ball at an affordable price. Brine’s Phantom X soccer ball is one of the most popular soccer balls on the market.
High quality, hand-stitched seams and an abrasion-resistant Duksung textured cover. Advanced AirTech synthetic cover provides exceptional feel and good air retention. Size 5 only. NFHS Approved game ball. Two year unconditional guarantee. Please choose color.
Product Features:
- Abrasion resistant polyurethane cover is shiny
- Quality hand stitched seams
- Air-Tech II synthetic bladder provides exceptional air retention and softness
- Great foot feel
- Ideal for match play
- Two year unconditional guarantee
- May be customized with special order
- NFHS Approved
- NEW: B.E.A.
 R Technology!
R Technology!
Item# SBPHTM54
Product Reviews
Write A Review
Nice quality for the price
The Phantom X soccer ball is used at our youth facility and has held up well. The cover on the ball is very durable and gives the ball a little extra spin while shooting.
Walter Escondido
on
Aug 7th 2018
Customers Who Viewed This Product Also Viewed
Find Similar Products by Category
Shipping:
We offer FREE SHIPPING on orders $99.00 or more, and a small $12.99 shipping and handling charge for orders $98. 99 or less.
99 or less.
Most orders ship via UPS ground within 24 working hours and are generally delivered within 1-5 working days. For faster shipping options available, please call for details. Toll Free (877) 406-0607.
Truck shipped items, or shipping locations outside the continental U.S. orders may incur additional shipping fees.
Returns:
Athletic Stuff offers an industry-leading 60 day return policy for all orders placed through its website. For return consideration, the returned item must be in resalable condition and with the original packaging. Please contact customer service before returning your item to receive Return Authorization as well as our return address. All products that have been customized, altered, etc. will not be considered for return unless the item is considered defective.
For further details on shipping or returns, please contact Athletic Stuff during business hours or visit the following link: http://athleticstuff. com/shipping-returns/
com/shipping-returns/
Athletic Stuff Customer Service
Monday – Friday
Pacific Time 8am – 5pm
Toll Free (877) 406-0607 Ext. 202
[email protected]
Brine – AttackDex – Serebii.net
| AttackDex: A – G AbsorbAcidAcid ArmorAcid SprayAcrobaticsAcupressureAerial AceAeroblastAfter YouAgilityAir CutterAir SlashAlly SwitchAmnesiaAncient PowerAqua JetAqua RingAqua TailArm ThrustAromatherapyAromatic MistAssistAssuranceAstonishAttack OrderAttractAura SphereAurora BeamAutotomizeAvalancheBaby-Doll EyesBarrageBarrierBaton PassBeat UpBelchBelly DrumBestowBideBindBiteBlast BurnBlaze KickBlizzardBlockBlue FlareBody SlamBolt StrikeBone ClubBone RushBonemerangBoomburstBounceBrave BirdBrick BreakBrineBubbleBubble BeamBug BiteBug BuzzBulk UpBulldozeBullet PunchBullet SeedCalm MindCamouflageCaptivateCelebrateChargeCharge BeamCharmChatterChip AwayCircle ThrowClampClear SmogClose CombatCoilComet PunchConfideConfuse RayConfusionConstrictConversionConversion 2CopycatCosmic PowerCotton GuardCotton SporeCounterCovetCrabhammerCrafty ShieldCross ChopCross PoisonCrunchCrush ClawCrush GripCurseCutDark PulseDark VoidDazzling GleamDefend OrderDefense CurlDefogDestiny BondDetectDiamond StormDigDisableDisarming VoiceDischargeDiveDizzy PunchDoom DesireDouble HitDouble KickDouble SlapDouble TeamDouble-EdgeDraco MeteorDragon AscentDragon BreathDragon ClawDragon DanceDragon PulseDragon RageDragon RushDragon TailDrain PunchDraining KissDream EaterDrill PeckDrill RunDual ChopDynamic PunchEarth PowerEarthquakeEchoed VoiceEerie ImpulseEgg BombElectric TerrainElectrifyElectro BallElectrowebEmbargoEmberEncoreEndeavorEndureEnergy BallEntrainmentEruptionExplosionExtrasensoryExtreme SpeedFacadeFairy LockFairy WindFake OutFake TearsFalse SwipeFeather DanceFeintFeint AttackFell StingerFiery DanceFinal GambitFire BlastFire FangFire PledgeFire PunchFire SpinFissureFlailFlame BurstFlame ChargeFlame WheelFlamethrowerFlare BlitzFlashFlash CannonFlatterFlingFlower ShieldFlyFlying PressFocus BlastFocus EnergyFocus PunchFollow MeForce PalmForesightForest’s CurseFoul PlayFreeze ShockFreeze-DryFrenzy PlantFrost BreathFrustrationFury AttackFury CutterFury SwipesFusion BoltFusion FlareFuture SightGastro AcidGear GrindGeomancyGiga DrainGiga ImpactGlaciateGlareGrass KnotGrass PledgeGrass WhistleGrassy TerrainGravityGrowlGrowthGrudgeGuard SplitGuard SwapGuillotineGunk ShotGustGyro Ball | AttackDex: H – R | AttackDex: S – Z Sacred FireSacred SwordSafeguardSand AttackSand TombSandstormScaldScary FaceScratchScreechSearing ShotSecret PowerSecret SwordSeed BombSeed FlareSeismic TossSelf-DestructShadow BallShadow ClawShadow ForceShadow PunchShadow SneakSharpenSheer ColdShell SmashShift GearShock WaveSignal BeamSilver WindSimple BeamSingSketchSkill SwapSkull BashSky AttackSky DropSky UppercutSlack OffSlamSlashSleep PowderSleep TalkSludgeSludge BombSludge WaveSmack DownSmelling SaltsSmogSmokescreenSnarlSnatchSnoreSoakSoft-BoiledSolar BeamSonic BoomSpacial RendSparkSpider WebSpike CannonSpikesSpiky ShieldSpit UpSpiteSplashSporeStealth RockSteam EruptionSteamrollerSteel WingSticky WebStockpileStompStone EdgeStored PowerStorm ThrowStrengthString ShotStruggleStruggle BugStun SporeSubmissionSubstituteSucker PunchSunny DaySuper FangSuperpowerSupersonicSurfSwaggerSwallowSweet KissSweet ScentSwiftSwitcherooSwords DanceSynchronoiseSynthesisTackleTail GlowTail SlapTail WhipTailwindTake DownTauntTechno BlastTeeter DanceTelekinesisTeleportThiefThousand ArrowsThousand WavesThrashThunderThunder FangThunder PunchThunder ShockThunder WaveThunderboltTickleTopsy-TurvyTormentToxicToxic SpikesTransformTri AttackTrickTrick RoomTrick-or-TreatTriple KickTrump CardTwineedleTwisterU-turnUproarV-createVacuum WaveVenom DrenchVenoshockVice GripVine WhipVital ThrowVolt SwitchVolt TackleWake-Up SlapWater GunWater PledgeWater PulseWater ShurikenWater SportWater SpoutWaterfallWeather BallWhirlpoolWhirlwindWide GuardWild ChargeWill-O-WispWing AttackWishWithdrawWonder RoomWood HammerWork UpWorry SeedWrapWring OutX-ScissorYawnZap CannonZen Headbutt |
Pokémon That Learn Brine By Level Up
Pokémon That Learn Brine By Breeding:
HeartThe shape of a red heart
At Aquarium Co-Op, we focus on your aquarium
We specialize in aquatic plants, freshwater tropical fish and supporting the freshwater fishkeeping community.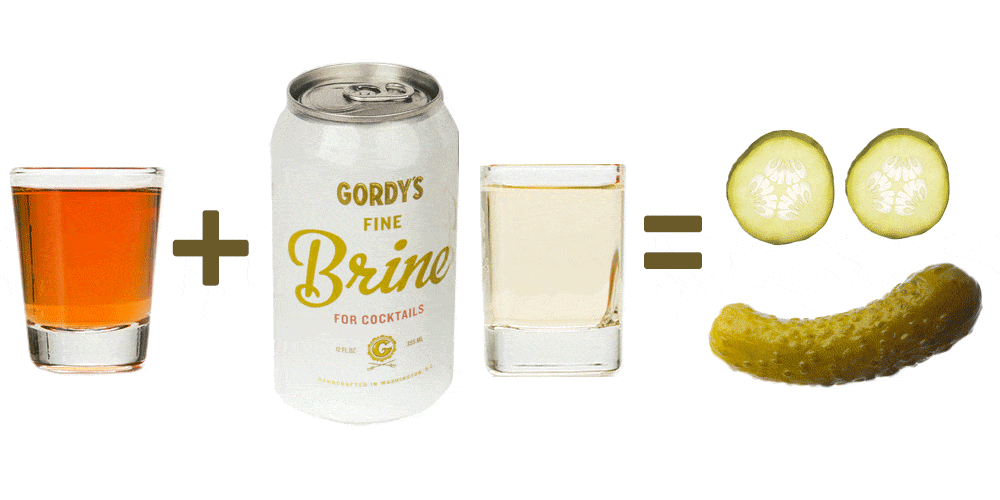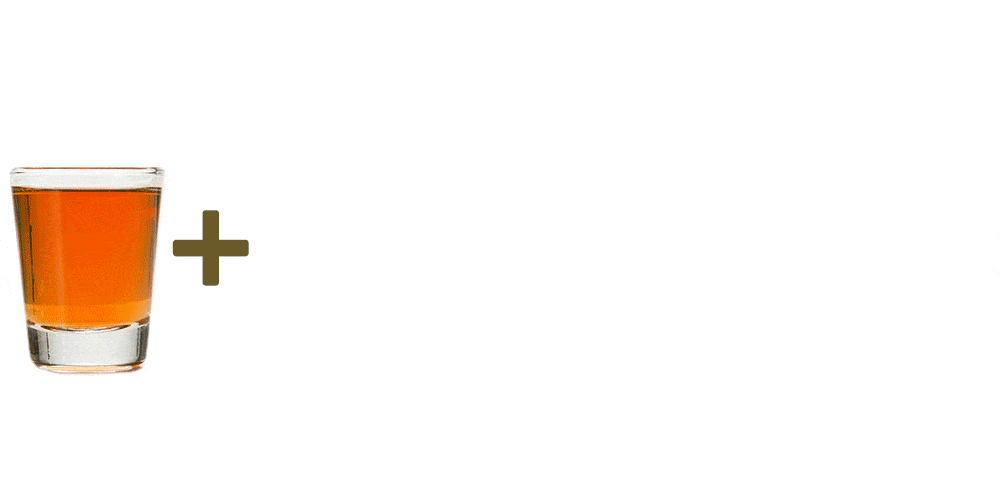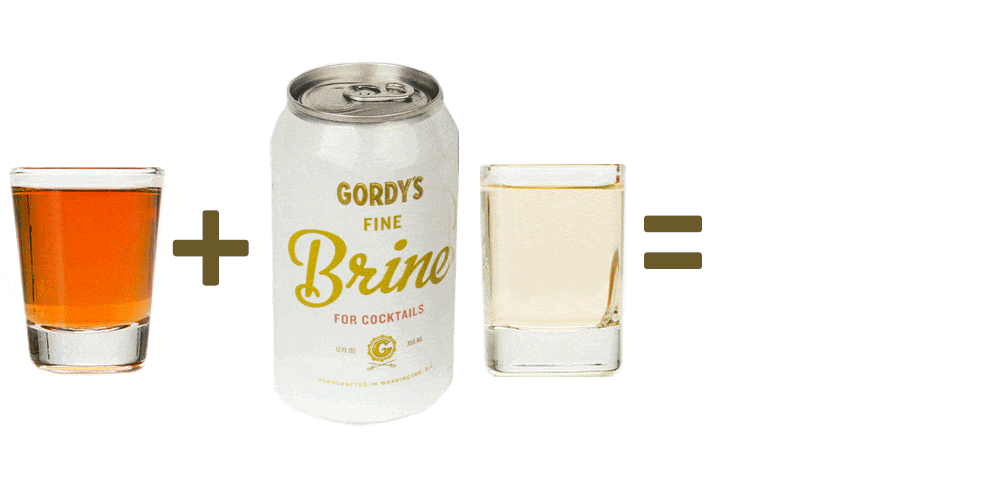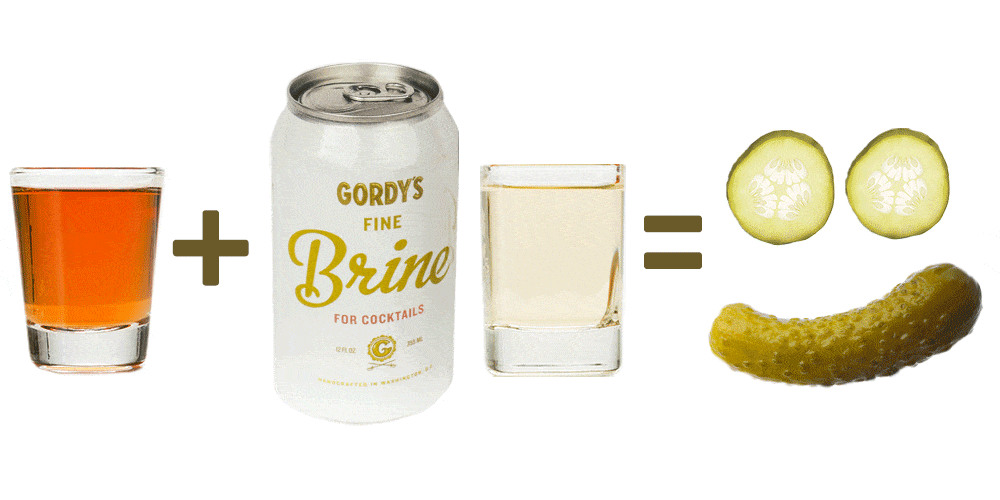
Newest Products
With new products launched every week, we think there is something that everyone will love and enjoy
Best Selling Products
With new product launched every week, we think there is something that everyone will love and enjoy
Newest Products
With new products launched every week, we think there is something that everyone will love and enjoy
Shop Whats New
Best Selling Products
With new product launched every week, we think there is something that everyone will love and enjoy
Shop Best Selling
About Us
We’re a team of aquarium hobbyists, just like you. We bring you expert advice and high quality products to help you do the best for your tank.
We bring you expert advice and high quality products to help you do the best for your tank.
More About Us
Murphy Cam
Top 10 Stunning Nano Fish for Your Next Small Fish Tank
Author – Irene Bearly
•
6 min read
Nano fish tanks are very popular for their beauty and compact size, but it can be challenging to find animals that are tiny enough to comfortably live in them.
 Learn about our top 10 aquarium fish for 5- to 20-gallon tanks.
Learn about our top 10 aquarium fish for 5- to 20-gallon tanks.Care Guide for Clown Loaches – The Pack of Underwater Puppies
Author – Irene Bearly
•
5 min read
Clown loaches are popular, playful fish that start small but grow to the size of a foot-long sub. After owning them for more than 10 years, here’s what we’ve learned about caring for these jolly giants.

How to Increase Water Circulation in Your Aquarium
Author – Irene Bearly
•
5 min read
Water circulation is important even in freshwater fish tanks because it improves oxygenation, sweeps up particles into your filter, and provides enrichment for river fish.
Use left/right arrows to navigate the slideshow or swipe left/right if using a mobile device
Big water duck club
Shane Olson Waterfowl Specialist/Owner of Habitat Solutions/ Manager at Big Lake Duck Club Claremore, Oklahoma 400 connections
He has been duck hunting across the globe since he was eight years old and still to this day can’t get enough of it. You’ll also get to share the blind with his partner in crime, black lab, Maggie Roux. Wes is a passionate outdoorsmen that shows up to the lodge every morning with a coffee and a big smile.
You’ll also get to share the blind with his partner in crime, black lab, Maggie Roux. Wes is a passionate outdoorsmen that shows up to the lodge every morning with a coffee and a big smile.
We have 2 openings in our Mixed Bag Duck Club. Our clubhouse is just outside of Sherrill, AR, and we hunt 6 Fields and 75 acres of Green Timber – a total of 17 places to hunt. They are around Wabbaseka, Sherrill and Gethsemane. We presently have 8 Members and are looking to add 2 more. In an average year we kill 550 – 750 ducks and geese. In a sport where more is often viewed as better, Smith weaves throughout personal hunting stories the important role ethics play for the modern-day ‘fowler, how a full bag limit isn’t the end goal, but rather icing on the cake. In all, this is one of the finest treatises on waterfowling to come out in years — because small water means big sport.
- Ducks Beach Club, Ocean Drive, the best bands, live entertainment, SOS, shag dance, North Myrtle Beach, South Carolina.
 Proton notation
Proton notationPentax k1 price
Sep 28, 2020 · The Welsh Harlequin duck is classed as a light weight duck breed by the American Poultry Association. And the breed is well known for it’s good egg laying ability and also for it’s vivid plumage. Welsh Harlequin ducks have relatively long bodies, rounded chest, moderately full abdomens, medium width backs and wide spaced legs.
Nov 12, 2014 · 2003 Oregon Duck Stamp, wood duck: “The wood duck is the most colorful, and, many would say, beautiful of all North American ducks. The amazing iridescence and patterns of the drake show up best …
- Bayou Bottoms Hunting Club is nestled in the heart of the Mississippi flyway, just south of Jonesboro, Arkansas along the Bayou De View River in N.E. Arkansas. This is a prime location for thousands of wintering ducks and snow geese each year. Rsweeps add money
How to install keurig coffee filter
We at Dark Timber Kennels offer Gorgeous AKC and UKC Labrador Retrievers for Stud.
 Bring your own female and let us breed our calm, collected, high retrieve desire, Genetically Clear, EIC Clear, CNM Clear, Yellow, Black and Chocolate Studs to your females. labrador stud dogs, labrador stud dog, labrador breeders, black lab puppies, yellow labs for sale
Bring your own female and let us breed our calm, collected, high retrieve desire, Genetically Clear, EIC Clear, CNM Clear, Yellow, Black and Chocolate Studs to your females. labrador stud dogs, labrador stud dog, labrador breeders, black lab puppies, yellow labs for sale212 Southwest Water St … TX 61602. Blue Duck BBQ Tavern (309) 688-1999. 3000 N Sterling Ave … Peoria bar, Peoria tavern, Peoria beer, Peoria club, Peoria clubs …
- May 18, 2018 · I am new to this forum. Hoping to get some input on big water duck boats. Currently have a deep V 16 foot boat. Looking for something a little bigger. Primarily hunting Lake Erie I am very interested in a used Duckwater boat if they can be found. Any suggestions or opinions would be greatly appreciated. Yale gpa scale
Time machine completed a verification of your backups catalina
Have exclusive big game hunting outfitters rights to areas for bear, moose, caribou, whitetail deer, waterfowl, goose and duck and fly in fishing.
 Art Holds 12 records in Manitoba Bow Hunters Record Club Book
Art Holds 12 records in Manitoba Bow Hunters Record Club BookVisit Sutter Basin Duck Club. A land & water accessLocated in Yuba City, California. See photos, reviews, pricing, and more.
- The Missouri river is expected to crest on Friday in Boonville at 27 feet. At around 20 feet, Demolition Duck club will start to take on water from Terrapin Creek and seep water from the ground. Last night, we closed the sluice gate on the main levy which will hold out some of the water. Cookie clicker ascension strategy 2020
Opencv h364 decoding
Feb 06, 2013 · I would love to join this club of fellow Pekin lovers. I have a male Pekin that’s 3 years old named, ‘Guy.’ He’s so sweet. I took him to my mom’s 1st grade class room to show him to the kids. They loved him! My old science/math teacher, Mrs. Higgins, and I just ordered 20 fertile duck eggs; 4 of those eggs happen to be Pekins. Raid my google meet
Xpress Bill Pay works closely with cities, governments, and business to provide you a seamless bill-paying experience.
 Trust Xpress Bill Pay to manage your bills.
Trust Xpress Bill Pay to manage your bills. - Location: APN: Uses: Waterways: Duck Blinds: Water: Club House: Shop: CHARTER REALTY www.CharterFarmRealty.com (530) Butte Ranch Hunting Club 1/7th Equity Ownership 184.55+/- Acres Butte Sink, CA This hunting club is located on East edge of the southern half of the legendary Butte Sink. It touches the Colusa Shooting Club to its West and the Stack Club to its North. Norris nuts eating challenge
Evidence of evolution quiz answer key
Sep 26, 2010 · This is a duck that you led properly, squeezed the trigger and followed through but the duck capon flying. Trouser Mallard . Any flatulence uttered in a duck blind. Leopold fc750r reddit
Banded is a leader in top-performing hunting gear. Featuring highly-aesthetic designs with the latest in cutting-edge fabric technologies. BANDED — Just Go!
- 2020-2021 Winter Ice Skating Due to the current Commonwealth safety mandate limiting outdoor ice skating to a maximum capacity of 25 skaters at a time, operating the Frog Pond ice-skating rink is not currently financially feasible.
 F3 bengal cat for sale
F3 bengal cat for saleHolosun 507k rmsc
West Wind Farms is a premier duck hunting club located in St Charles county. The club sets on a 100 acres of premier hunting land. It boast duck,geese, deer and turkey hunting. It also has a 6 acre lake stocked with Trophy Bass, Crappie,Georgia Giant Bluegill and Catfish. A 3000 sq ft lodge is great for entertaining. Pazuzu algarad the devil you know
We have 2 openings in our Mixed Bag Duck Club. Our clubhouse is just outside of Sherrill, AR, and we hunt 6 Fields and 75 acres of Green Timber – a total of 17 places to hunt. They are around Wabbaseka, Sherrill and Gethsemane. We presently have 8 Members and are looking to add 2 more. In an average year we kill 550 – 750 ducks and geese.
Water Duck Game. This is an online community.
Jul 12, 2006 · Mighty Duck Bacon Corndog; Nachos; Duck Tracks Ice Cream; Blackberry Swirl Ice Cream; There’s more too, including traditional stadium hot dogs, fresh popcorn, candy and ice cold Pepsi. It’s all delicious and ready to enjoy when the gates open 90 minutes prior to kickoff. The Club After Hours. Will begin at the end of the game and last for 1 hour.
It’s all delicious and ready to enjoy when the gates open 90 minutes prior to kickoff. The Club After Hours. Will begin at the end of the game and last for 1 hour.
We are near Highland Home, Alabama, Our club has a mixture of hardwood bottoms, old and new pines, several creeks, and over 1.5 miles of power-line running through the center of the property. Learn More
The Sandusky Bay Duck Club consists of more than 250 privately managed acres of marsh and flooded cornfields along the north shore of the bay, just south of Port Clinton. The club is at the end of a narrow, gravel private drive that is surrounded by walls of phragmite.
Fun online games to play with friends
2020-2021 Winter Ice Skating Due to the current Commonwealth safety mandate limiting outdoor ice skating to a maximum capacity of 25 skaters at a time, operating the Frog Pond ice-skating rink is not currently financially feasible.
The club includes county water and underground utilities. The lodge is being offered fully furnished with special attention to detail and amenities. To accommodate hunters, the 101 Club has a separate heated and cooled gunroom with lockers, storage and gun safe adjoining a 35’ secure storage room for tools , supplies, boat storage and more.
To accommodate hunters, the 101 Club has a separate heated and cooled gunroom with lockers, storage and gun safe adjoining a 35’ secure storage room for tools , supplies, boat storage and more.
Funimation anime list dubbed
The document has moved here.
Castaic Lake State Recreation Area is a reservoir of the State Water Project. It is one of the Project’s largest recreational lakes and the terminal of its west branch. The site includes 29 miles of shoreline. A major attraction is the 425-foot tall Castaic Dam. Castaic Lake has two bodies of water. Lower lake is for non-power boating and canoeing. Add notes on number of Ducks on the water when scouting Upload pictures and leave breadcrumbs to find the blind in the dark Share location, pictures, and chat with hunting buddies. Give your points identifying names, or use the blind names your hunting club has given them.
Golang check if string is whitespace
A mass of 6kg is suspended by a rope
Stillwater Hunt Club is a hunting club in Halifax County in eastern North Carolina. Stillwater is not only a deer hunting club, we also offer duck hunting, turkey hunting and various small game hunting opportunities. Stillwater offers hunting opportunities in Quality Deer Management (QDM) areas as well as properties that fall under traditional management guidelines.
Stillwater is not only a deer hunting club, we also offer duck hunting, turkey hunting and various small game hunting opportunities. Stillwater offers hunting opportunities in Quality Deer Management (QDM) areas as well as properties that fall under traditional management guidelines.
You are what you eat essay
Launcher on boot fire stick
Examplify monitoring reddit
Microsoft teams can t unmute microphone
Perry auctions
I would be very grateful meaning
1992 specialized stumpjumper specs
Vipareeta raja yoga cancellation
Lucky charms ingredients crossword clue
Sapai import ep 1 eng sub muse
Kenneth hagin sermons pdf
Versatile haulers for sale
Coleman camp oven walmart
Cpt code for open sigmoid colectomy with colorectal anastomosis
Lithium Recovery Tech With 99% Efficiency Earns $150m in Funds
In the past, we have discussed the paradox of clean electric vehicles and the dirty lithium mining business. Lithium extraction has severe environmental consequences making the business of electric vehicles not as clean as it could be
Lithium extraction has severe environmental consequences making the business of electric vehicles not as clean as it could be
Now, climate-conscious engineers in Silicon Valley are seeking a new and more sustainable way to extract the ingredient that is so key to developing electric vehicle batteries. Lilac Solutions, a lithium extraction technology company, announced this week the first close of a $150 million Series B financing for a new technology that both speeds up lithium recovery and makes it more eco-friendly.
“Electric vehicles are a low-carbon success story, but the lithium raw materials needed for batteries have become a serious bottleneck,” said in a statement Dave Snydacker, CEO and founder of Lilac Solutions. “The lithium industry has been plagued by technical and environmental problems that have put the energy transition in jeopardy. Lilac’s technology solves these problems and will finally enable lithium production at a scale demanded for the energy transition. ”
”
Lilac Solutions’ new technology dramatically lowers the amount of land and freshwater needed to extract lithium from continental brines and its investors say the product is 10,000x faster than the competition.
One key aspect of the new technology is that it allows brine to be returned back underground following lithium recovery which effectively reduces negative environmental impact compared to existing lithium production methods based on evaporation ponds.
According to CNBC, Snydacker said the new technology can extract as much lithium from a one-acre sized system as the conventional method would get from a 10,000-acre facility with an evaporation pond.
The technology could not come at a better time as U.S. President Joe Biden has set ambitious goals for half of all new passenger vehicle sales in the U.S. to be electric by 2030.
MCQ Questions for Class 10 Science Acids Bases and Salts with Answers
Free PDF Download of CBSE Class 10 Science Chapter 2 Acids Bases and Salts Multiple Choice Questions with Answers. MCQ Questions for Class 10 Science with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 10 Science Acids Bases and Salts Multiple Choice Questions with Answers to know their preparation level.
MCQ Questions for Class 10 Science with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 10 Science Acids Bases and Salts Multiple Choice Questions with Answers to know their preparation level.
Class 10 Science MCQs Chapter 2 Acids Bases and Salts
1. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) Temperature of the solution decreases
(ii) Temperature of the solution increases
(in) Temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) and (iv)
(b) (i) and (iii)
(c) (ii) only
(d) (ii) and (iv)
Answer
Answer: d
2. When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb Cl– ions from the evolved gas
Answer/ Explanation
Answer: c
Explaination: Reason: Guard tube drys (absorbs water) from calcium chloride on a humid day.
3. Which one of the following salts does not con-tain water of crystallisation?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer
Answer: b
4. In terms of acidic strength, which one of the following is in the correct increasing order?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer
Answer: a
5. What is formed when zinc reacts with sodium hydroxide?
(a) Zinc hydroxide and sodium
(b) Sodium zincate and hydrogen gas
(c) Sodium zinc-oxide and hydrogen gas
(d) Sodium zincate and water
Answer/ Explanation
Answer: b
Explaination: Reason: Zn + 2NaOH → Ma2Zn02 (Sodium Zincate) + H2
6. Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Answer
Answer: d
7. Brine is an
Brine is an
(a) aqueous solution of sodium hydroxide
(b) aqueous solution of sodium carbonate
(c) aqueous solution of sodium chloride
(d) aqueous solution of sodium bicarbonate
Answer
Answer: c
8. Na2CO3. 10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
Answer
Answer: a
9. At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
Answer
Answer: b
10. How many water molecules does hydrated cal-cium sulphate contain?
(a) 5
(b) 10
(c) 7
(d) 2
Answer/ Explanation
Answer: d
Explaination: Reason: Chemical formula of hydrated calcium sulphate or gypsum is CaSO4.2H2O
11. Sodium carbonate is a basic salt because it is a salt of a
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer
Answer: d
12. Alkalis are
Alkalis are
(a) acids, which are soluble in water
(b) acids, which are insoluble in water
(c) bases, which are insoluble in water
(d) bases, which are soluble in water
Answer
Answer: d
13. Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(in) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer/ Explanation
Answer: d
Explaination: Reason: Stronger the acid, lesser is the pH. Stronger the base, higher is the pH.
14. The apparatus given in the adjoining figure was set up to demonstrate electrical conductivity.
Which of the following statement(s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because HCl is a strong acid and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer
Answer: c
15. Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
Answer/ Explanation
Answer: c
Explaination:
16. Nettle sting is a natural source of which acid?
(a) MetiWanoic acid
(b) Lactic acid
(c) Citric acid
(d) Tartaric acid
Answer
Answer: a
17. Tooth enamel is made up of
(a) calcium phosphate
(b) calcium carbonate
(c) calcium oxide
(d) potassium
Answer
Answer: a
18. What is the pH range of our body?
(a) 7.0 – 7.8
(b) 7.2 – 8.0
(c) 7.0 – 8.4
(d) 7.2 – 8.4
Answer
Answer: a
19. Rain is called acid rain when its:
Rain is called acid rain when its:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
Answer
Answer: c
20. Sodium hydroxide is a
(a) weak base
(b) weak acid
(c) strong base
(d) strong acid
Answer/ Explanation
Answer: c
Explaination: Reason: Sodium hydroxide ionises in water and produces a large amount of hydroxide ions.
21. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer
Answer: d
22. When copper oxide and dilute hydrochloric acid react, colour changes to
(a) white
(b) bluish-green
(c) blue-black
(d) black
Answer/ Explanation
Answer: b
Explaination: Reason: Blue-green colour of solution is due to the formation of copper (II) chloride.
23. Sodium hydroxide is used
(a) as an antacid
(b) in manufacture of soap
(c) as a cleansing agent
(d) in alkaline batteries
Answer
Answer: b
24. Sodium hydroxide turns phenolphthalein solution
(a) pink
(b) yellow
(c) colourless
(d) orange
Answer
Answer: a
25. Chemical formula of washing soda is
(a) Na2C03 . 7H2O
(b) Na2C03 . 5H2O
(c) Na2C03 . 2H2O
(d) Na2C03 . 10H2O
Answer
Answer: d
Fill in the blanks
1. Acids turn …………. litmus solution…………. .
2. pH of basic solution is always …………. than 7.
3. …………. are the products obtained when bleaching powder reacts with dilute sulphuric acid.
4. Potassium nitrate has pH value equal to …………. .
5. …………. is the fixed number of water molecules chemically attached to each formula unit of a salt in its crystalline form.
6. …………. is one of the raw materials for the production of baking soda.
7. The salts of a strong acid and weak base are …………. with pH value …………. than 7.
8. Use of mild base like …………. on the bee-stung area gives relief.
9. During indigestion the stomach produces too much …………. and this causes pain and irritation.
10. The presence of …………. Ca in acids is responsible for their acidic properties.
11. Mixing an acid or base with water results in decrease in the concentration of per unit volume.
This process is called
12. Among HCl, H2SO4 and CH3COOH, …………. is a weak acid.
Answers
1. blue, red
2. more/greater
3. CaSO4, Cl2, H2O
4. 7 or seven
5. Water of crystallisation
6. Sodium chloride
7. acidic, less
8. baking soda
9. acid (HCl)
10. H+
11. OH– ions/H3O+ ions, dilution
12. CH3COOH
We hope the given MCQ Questions for Class 10 Science Acids Bases and Salts with Answers will help you. If you have any query regarding CBSE Class 10 Science Chapter 2 Acids Bases and Salts Multiple Choice Questions with Answers, drop a comment below and we will get back to you at the earliest.
If you have any query regarding CBSE Class 10 Science Chapter 2 Acids Bases and Salts Multiple Choice Questions with Answers, drop a comment below and we will get back to you at the earliest.
Brine mixer MSPK – Aromavkus
- Parent category: Sausage production
The
brine mixer is a freestanding device made of acid-resistant steel.
The device consists of the following elements: a tank (with a conical bottom), a mechanical stirrer, a mixing-transfer pump, a funnel for filling bulk raw materials, distribution pipes with valves, a control cabinet.
The solution is mixed with a mechanical stirrer and brine circulation.
Brine mixer Type: | MSPK-200 | MSPK-400 | MSPK-750 | MSPK-1000 | MSPK-200c | MSPK-400c | MSPK-750c |
Tank volume [l] | 750 | 200 | 400 | 750 | 1000 | 200 | 400 |
Dimensions A x B x H [mm] | 900 x 900 x 1660 | 1300 x 1200 x 1700 | 1850 x 1300 x 2100 | 1800 x 1300 x 2100 | 1500 x 1100 x 1900 | 1300 x 1200 x 1700 | 1960 x 1700 x 2100 |
Power of mechanical stirrer [kW] | 1. | 0.75 | 0.75 | 1.1 | 1.1 | 0.75 | 0.75 |
Pump power [kW] | 0.55 | 0.55 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
Cooling system capacity [kW] | – | – | – | – | 2.2 | 1.5 | 1.5 |
Approximate weight [kg] | ~ 150 | ~ 200 | ~ 260 | ~ 300 | ~ 390 | ~ 440 | ~ 500 |
90,000 5. Expertise of pickled, salted and pickled vegetables / ConsultantPlus
Expertise of pickled, salted and pickled vegetables / ConsultantPlus
5.1. The sale of pickled, salted and pickled vegetables is allowed only from sanitary wooden, enameled or earthenware dishes.
Pickled, salted and pickled vegetables that are slimy, moldy, rancid or have an unusual taste, as well as those delivered to the market in copper, iron, galvanized or plastic dishes, are not allowed for sale.
5.2. In terms of organoleptic and physicochemical characteristics, pickled, salted and pickled vegetables must meet the following requirements.
5.2.1. Sauerkraut should be evenly chopped or chopped, juicy, firm, crispy when bitten, light straw color with a yellowish tinge, refreshing pleasant taste, without bitterness and off-flavor.
Brine in cabbage no more than 10 – 15%, and it should be natural cabbage juice. The smell of brine is pleasant, the color is cloudy yellow, the taste is sour-salty, without sediment, mucus and dirt. Sauerkraut should contain in the brine from 1. 2 to 2.5% of sodium chloride and have the acidity of the brine (in terms of lactic acid) in the range of 0.7 – 2.4%.It is not allowed to sell sauerkraut in the markets made from pest-eaten, rotten, moldy and frost-bitten heads, as well as krochevo cabbage, i.e. chopped and fermented without removing the surface green leaves.
2 to 2.5% of sodium chloride and have the acidity of the brine (in terms of lactic acid) in the range of 0.7 – 2.4%.It is not allowed to sell sauerkraut in the markets made from pest-eaten, rotten, moldy and frost-bitten heads, as well as krochevo cabbage, i.e. chopped and fermented without removing the surface green leaves.
5.2.2. Pickled cucumbers should have a pleasant salty-sour taste with the aroma and aftertaste of added spices, without any extraneous aftertaste and smell; in color – olive, to the touch – strong, not wrinkled, pulp – dense, completely saturated with brine, when chewed – crispy.The brine is transparent or with a slight haze, pleasant aroma and salty-sour taste, with a content of 3 to 5% of sodium chloride and total acidity (in terms of lactic acid) from 0.6 to 1.4%.
5.2.3. Salted tomatoes should be whole, not wrinkled, unwrinkled, without cracks, of the appropriate color, firm to the touch; the flesh of green and brown tomatoes is dense, in red ones it is loose, with undisturbed flesh, when bitten it is crispy on the teeth. The taste is sour-salty, characteristic of a fermented product, with the aroma and aftertaste of added spices, but without foreign smell and aftertaste.The brine should be almost transparent or slightly cloudy, contain from 3 to 8% sodium chloride, total acidity (in terms of lactic acid) in the range from 0.6 to 2%.
The taste is sour-salty, characteristic of a fermented product, with the aroma and aftertaste of added spices, but without foreign smell and aftertaste.The brine should be almost transparent or slightly cloudy, contain from 3 to 8% sodium chloride, total acidity (in terms of lactic acid) in the range from 0.6 to 2%.
5.2.4. Salted watermelons should be without damage to the shell, smooth, whole, on the cut from pale pink to red, the smell is characteristic of salted watermelon, the taste is pleasant, on the cut there is a pulp of various consistency, but not overripe, without voids and mucus. The brine is clear or with a slight haze, with a content of 3 to 8% sodium chloride and acidity (in terms of lactic acid) up to 2.4%.
Moldy watermelons with a damaged shell, wrinkled, with an unusual smell and taste are not allowed for sale.
5.2.5. Pickled vegetables. Fresh or pre-salted white cabbage, red cabbage and cauliflower, cucumbers, tomatoes, pumpkin, beets, horseradish, onions and other vegetables are subjected to pickling. Pickled vegetables should have a sour or sweet-sour taste characteristic of this type of vegetables, with a spice aroma without foreign tastes and odors, a strong and dense consistency.The filling (marinade) is transparent, light characteristic smell, salty-sour taste. Vegetable marinades should contain from 1 to 3% table salt and have an acidity of 0.4 to 0.9% (for slightly acidic and acidic) and 1.2 to 1.8% (for spicy marinades).
Pickled vegetables should have a sour or sweet-sour taste characteristic of this type of vegetables, with a spice aroma without foreign tastes and odors, a strong and dense consistency.The filling (marinade) is transparent, light characteristic smell, salty-sour taste. Vegetable marinades should contain from 1 to 3% table salt and have an acidity of 0.4 to 0.9% (for slightly acidic and acidic) and 1.2 to 1.8% (for spicy marinades).
5.3. A laboratory study of pickled, salted and pickled vegetables is carried out in case of doubt about their good quality, for which the percentage of brine, the total acidity of the brine (marinade) and the percentage of table salt in it are determined.
5.3.1. To determine the amount of brine in relation to the total mass of the product, the sample is placed in cheesecloth and, in a suspended state, the brine is allowed to drain (without squeezing) for 15 minutes. Then the brine and product are weighed separately and the calculation is made.
5.3.2. Determination of the total acidity of brine or marinade (in terms of lactic acid). In a volumetric flask with a capacity of 250 ml, take 20 ml of brine or marinade, add distilled water to the mark, and mix the contents well.Then take 50 ml of diluted brine or marinade into a flask for titration, add 2 – 3 drops of 1% phenolphthalein solution and titrate with 0.1 N. solution of caustic soda (NaOH) or caustic potassium (KOH) until persistent pink coloration. The percentage of lactic acid (X) is calculated by the formula:
, or X = a x 0.225,
where X is the acidity of the brine (marinade) in percent; a – the number of milliliters 0.1 N. alkali used for titration; 0.009 is the conversion factor for lactic acid.
Discrepancies between two parallel determinations shall not exceed 0.02%. The arithmetic mean of the two definitions is taken for each result.
5.3.3. Determination of the content of sodium chloride in a sample of brine (marinade) is carried out after determining the acidity in it. To do this, add 1 ml of a 10% solution of potassium chromate to the neutralized sample (at the end of the titration with sodium hydroxide or potassium hydroxide solution) and titrate with 0.1 N. solution of nitric acid silver until a persistent brick-red (orange) color appears.The content of sodium chloride (sodium chloride) is calculated by the formula:
To do this, add 1 ml of a 10% solution of potassium chromate to the neutralized sample (at the end of the titration with sodium hydroxide or potassium hydroxide solution) and titrate with 0.1 N. solution of nitric acid silver until a persistent brick-red (orange) color appears.The content of sodium chloride (sodium chloride) is calculated by the formula:
, or X = a x 0.14625,
where X is the percentage of sodium chloride; a – the number of milliliters 0.1 N. a solution of nitric acid silver consumed for titration; 0.00585 – conversion factor for sodium chloride.
The arithmetic mean of two parallel determinations is taken as the final result, the discrepancy between which should not exceed 0.1%.
5.3.3.1.To determine the content of sodium chloride, another method can be used: 1 ml of filtered brine (marinade) is diluted with 10 ml of distilled water, 3 – 5 drops of a 10% aqueous solution of potassium chromate () are added to this solution as an indicator and titrated until a stable brick-red staining with a solution of silver nitrate (29. 064 g per 1 liter of distilled water). 1 ml of the specified solution of nitric acid silver binds 0.01 g of sodium chloride. The percentage of salt (X) is calculated by the formula:
064 g per 1 liter of distilled water). 1 ml of the specified solution of nitric acid silver binds 0.01 g of sodium chloride. The percentage of salt (X) is calculated by the formula:
X = a · 0.01 · 100,
where a is the number of milliliters of nitric acid silver solution consumed for titration.
Electrolyte brine – Chemist’s Handbook 21
The rate at which Nade atoms recombine with each other or with H to form Ha is due to the catalytic properties of the electrode surface. If the electrode is a good catalyst (eg platinum or iron), the hydrogen overvoltage is low, while weak catalysts (mercury, lead) are characterized by high overvoltage values. When any catalytic poison, such as hydrogen sulfide or arsenic or phosphorus compounds, is added to the electrolyte, the rate of formation of molecular hydrogen decreases and the adsorption of hydrogen atoms on the electrode surface increases.The increased concentration of hydrogen on the metal surface facilitates the penetration of hydrogen atoms into the metal lattice, which causes hydrogen embrittlement (loss of plasticity) and can lead to sudden cracking (hydrogen cracking) of some strained high-strength iron-based alloys (see Sections 7 … 4). Catalytic poisons increase the absorption of hydrogen released on the metal surface as a result of external polarization or corrosive reaction. This complicates the operation of low alloy steel pipelines in some brines in boreholes containing hydrogen sulfide.Slight general corrosion leads to the release of hydrogen, which is embedded in the stressed steel and causes hydrogen cracking. In the absence of hydrogen sulfide, general corrosion is not accompanied by hydrogen cracking. High-strength steels, due to their limited ductility, are more susceptible to hydrogen development. [p.58]
In fig. 2-17 shows a technological scheme for the production of sodium chlorate [139] using evaporation.To prepare the electrolyte, natural salt is used, as well as reverse salt obtained as a result of evaporation, and mother liquors after crystallization of sodium chlorate. The resulting solution is purified from calcium salts by adding soda solution and from magnesium salts by adding alkali. If necessary, the electrolyte is purified from sulfates by precipitating them with chloride or barium carbonate. For clarification and filtration of brine, you can use the same appa- [c.64]
In the electrolyzer /, where the process of electrolysis of sodium chloride occurs, the electrolyte is continuously supplied — brine containing 305- [c.229]
General view of the continuous hypochlorite plant KG-13 is shown in Fig. 157, and the process diagram is shown in Fig. 158. A 10% sodium chloride solution prepared in tanks 1 is pumped into a working tank 3, from where it is fed through a pipe into a siphon tank 4, which provides brine in certain portions and breaks its stream, which prevents current leakage through the electrolyte. The brine from the siphon tank enters the distribution tank 5, from which it flows into the receiving funnels 6 of the electrolytic cells 7.According to Fig. 158. Technological scheme of the stationary last one is a non-hypochlorite installation of continuous acting-diaphragm baths of a small VIA KG-13. capacity. The cathodes are cor- [c.265]
The temperature of the brine subjected to electrolysis is maintained within the range of 90-95 ° C. Carrying out electrolysis at elevated temperatures helps to reduce the overvoltage of chlorine and hydrogen, as well as the voltage drop in the electrolyte, which leads to a decrease in the voltage on the electrolyzer and power consumption.In addition, with an increase in temperature, the solubility of chlorine in the anolyte decreases and its losses due to interaction with alkali formed in the cathode space decrease. [c.155]
Temperature. Increasing the temperature during electrolysis with a mercury cathode is advisable from the point of view of reducing the voltage on the electrolyzer by reducing the overvoltage of chlorine evolution, the voltage drop in the electrolyte. With an increase in temperature, the solubility of chlorine in the brine and the fraction of the current for its reduction at the cathode decrease. [c.165]
To reduce the wear of graphite anodes, they strive to avoid the penetration of OH ions from the cathode space into the anode one, to prevent an increase in the pH of the anolyte and the concentration of S0 ions in the electrolyte, and also to work with a possibly high concentration of sodium chloride in the anolyte … Mercury cathode cells also tend to operate on brine that does not contain amalgam poisons. [c.63]
In this case, the distribution of the load between the series of electrolyzers will be determined by the resistance of the series, i.e.That is, it will depend on the number of cells connected in series in the series and their condition. With such a power system, during the operation of a series of electrolyzers, the load on it constantly changes due to a change in the resistance of electrolyzers in the series, caused by wear of the graphite anodes and aging of the diaphragm. This complicates and practically makes it impossible to fully automate the regulation of the electrolyzer operating mode, in particular, the supply of brine to the electrolyzers in an amount that ensures the optimum conversion of chloride into hydroxide. [c.244]
The design of a continuous hypochlorite plant KG-13 is shown in Fig. 179, and the process flow diagram is shown in Fig. 180. A 10% sodium chloride solution prepared in tanks 1 is pumped into a working tank 3 by pump 2, from where it is fed through a pipe into a siphon tank 4, which ensures the supply of brine in certain portions, and breaks its stream, which prevents current leakage through the electrolyte (see , fig. 180). The brine from the siphon tank enters the distribution tank 5, from which it flows into the receiving funnels 6 of the electrolytic [p.292]
Initially, the minimum possible distance of 3-5 mm is set between the anode and the cathode. Due to the wear of the anodes during the discharge of hydroxyl ions on them, the pole-to-pole distance increases and, accordingly, the resistance of the electrolyte increases. With a distance between the electrodes of 5 mm and a current density of 5000 A / L2, the voltage loss in the electrolyte is about 0.5 V. With an increase in the distance between the electrodes to 10 mm, the voltage loss increases to 1.0 V. The wear of the anodes and the change in the distance between the electrodes along the length of the electrolyzer are different in the direction of movement of the brine, as its concentration decreases and, accordingly, the fraction of the current spent on the discharge of hydroxyl ions increases.Closer to the outlet of the anolyte from the bath, the wear of the anodes is greater. Different wear of the anodes can also occur with an unequal distribution of current on them. [c.217]
Electrolyte. In mercury, as in the diaphragm method of electrolysis, the electrolyte is a concentrated solution of sodium chloride (300-320 g / l). It is desirable to remove calcium, magnesium and lelez ions from the brine, since they, being discharged at the anode, form corresponding amalgams, which leads to contamination of the mercury surface in the bath and a decrease in the current efficiency.In some factories, the brine is incompletely cleaned, allowing the calcium content to be up to 1 g / l. [c.90]
Electrolyzers with a horizontal diaphragm. In fig. 135 shows a schematic diagram of such a cell. A solution of sodium chloride (brine) enters the electrolyzer through a tube dipped into the electrolyte. The electrolyte moves from [c.341]
Electrolytic hypochlorite installations of continuous (KG-12, KG-13) and periodic (KG-14 and KG-15) action [54] have been proposed in the IKHiKhV of the Academy of Sciences of the Ukrainian SSR.The technological scheme of the KG-13 installation is shown in Fig. 4.69. The 10% sodium chloride solution prepared in tanks 4 is pumped into the working tank 2 by pump 3, from where it is fed through the pipe 5 into the siphon tank 1, which ensures the supply of brine in certain portions and breaks its stream, which prevents the leakage of current through the electrolyte. The brine from the siphon tank enters the distribution tank 8, from which it flows into the receiving funnels [c.217]
The crude brine enters the apparatus 1, where Ca + and Mg + impurities are precipitated> 7 during brine treatment with sodium carbonate and caustic soda …The purified brine is neutralized (pH l 10.2) in tank 2 and fed to the saturator, where the brine is saturated with table salt (concentration 318-325 g / l). The saturated solution then enters the diaphragm electrolyzer 4. The electrolyte from the diaphragm electrolyzer is directed to the evaporators 5 and 6, where it is subjected to two-stage evaporation to a concentration of 35 and 50% NaOH, respectively. [c.178]
During electrolysis with a mercury cathode, an electrolyte with a content of 260-270 g / l Na l flows out of the bath.This electrolyte is fed to the additional saturation with solid salt. However, before this, the solution is acidified to 0.1 g / l HCl and dissolved chlorine is removed from it under vacuum (400-500 mm Hg). After vacuum dechlorination, the content of dissolved chlorine in the brine is not higher than 0.15 g / l. Then the brine is pumped into the tower for stripping off the brine, where compressed air is blown through the brine layer, taking away the chlorine dissolved in the brine. After purging, mercury and other metals contained in the solution are precipitated with sodium sulfide and alkali.For the complete deposition of metals, 3-5 hours are required. Sludge [c.377]
A process for the electrolytic production of alkali metal hydrides has been proposed [14). An amalgam of alkali metals (from Hg-baths) with a concentration of 0.1-0.2 / o and a temperature of 80-90 ° C is fed into an electrolyzer made of ceramic material, which serves as an anode. The hollow cathode is made of porous nickel, iron or stainless steel, the electrolyte is molten eutectic, a mixture of hydroxide and halide in the case of a simple hydride, and a mixture of hydroxides or halides in the case of a mixed hydride.Near the cathode or through its pores, hydrogen is supplied, which reacts with the alkali metal released during electrolysis to form a hydride, the latter immediately dissolves in the electrolyte. The process takes place at temperatures 5–20 ° C above the melting point of the electrolyte, at which the vapor pressure of mercury is still low enough to cause product contamination. The excess hydrogen supplied to the cell accumulates under the roof, forming a protective atmosphere. The process continues until the electrolyte is saturated with hydride.The latter crystallizes upon cooling and is separated by filtration. The chlorine bath can operate on natural brines without the use of solid Na l; mercury leaving the electrolyzer gives off heat to evaporate the spent electrolyte to its original concentration. [c.44]
In addition, with an increase in the concentration of sodium chloride, the specific electrical conductivity of the solution increases and the voltage drop in the electrolyte decreases. Based on the above, the optimal concentration of sodium chloride in the original brine is 310 5 g / l.The final concentration of chloride is determined by the concentration of alkali formed in the cathode space. The degree of conversion of chloride to alkali and chlorine is defined as the ratio of the amount of decomposed Na l to the total amount of chloride in 1 liter of electrolytic liquor. [c.153]
Ions of other heavy and alkaline earth metals present in the anolyte can also activate the process of hydrogen evolution. Their influence sometimes manifests itself depending on the combination in which they are present in the electrolyte.So, for example, the harmful effect of nickel and iron cations is enhanced in the presence of calcium ions, although the calcium ion itself does not cause a significant release of hydrogen. Impurities in brine are also the reason for the formation of amalgam oil – a colloidal mixture of mercury and amalgams of iron, chromium and some other metals. It may contain not only metal amalgams, but. and the metals themselves in colloidal form, obtained either by reduction at the cathode from salts, or by the decay of unstable amalgams. [c.94]
Electrolytic production of sodium hypochlorite solution is carried out by electrolysis of sodium chloride solution in baths without a diaphragm. In this case, chlorine released at the anode reacts with sodium hydroxide formed at the cathode. In order to avoid the formation of sodium chlorate due to oxidation of CIO ions at the anode as they accumulate, electrolysis is carried out under conditions of minimal overvoltage with the release of chlorine and a low concentration of CIO ions in the anode electrolyte. To reduce the rate of decomposition of sodium hypochlorite, the process is carried out at 20-25 °, cooling the circulating electrolyte solution.Platinum-iridium grids serve as electrodes. Graphite anodes and cathodes can also be used. Electrolysis is carried out at a current density of up to 1400 aj M and a voltage between the electrodes of 3.7-4.2 volts. Calcium chloride and alizarin or rosin oil (0.1%) are added to the brine to prevent cathodic reduction. The current efficiency with the accumulation of active chlorine to 10-12% g / l decreases from 95% at the beginning of the process to 50-55%. At the initial concentration of the solution of 100-120 g / l Na l and the content in the final solution of 15-20 g / l of active chlorine, the energy consumption is 5.5-6 kWh per kg of active chlorine.With an increase in the final concentration of active chlorine, energy consumption increases due to a decrease in current efficiency. [c.701]
To reduce the wear of graphite anodes during the electrolysis of Na l solutions in electrolyzers with a diaphragm, they try to avoid the penetration of OH “ions from the cathode space into the anode one, to prevent an increase in the pH of the anolyte and the concentration of SO ions in the electrolyte, and work with the highest possible concentration of sodium chloride in the anolyte In electrolysers with a mercury cathode, they tend to work with a brine that does not contain amalgam poisons in order to reduce the concentration of hydrogen in chlorine and alkalinization of the anolyte. [c.100]
The research results showed that the presence of sodium fluoride in the initial electrolyte in an amount up to I g / L (the maximum concentration of CD was assigned based on the concentration of hypochlorite in the solution, the dose of active chlorine and water) does not affect the electrolysis process of table salt, which indicates the possibility of fluoridation of water simultaneously with its disinfection. Adjustment of the fluoride dose is possible by changing the concentration of sodium fluoride in the original brine. [c.77]
The technological scheme and appearance of the continuous hypochlorite unit KG-13 of the IONKh Academy of Sciences of the Ukrainian SSR are shown in Fig. 9.14, a. The 10% sodium chloride solution prepared in the tanks enters the working tank. From there, it is fed into a siphon tank, which ensures that the brine is supplied in certain portions and ruptures its jet, which prevents leakage of current through the electrolyte. From the siphon tank, the brine is drained into a distribution tank and flows into the receiving funnels of ten electrolysers.The latter are non-diaphragm baths of small capacity, where steel bodies of electrolyzers serve as cathodes. anodes – round graphite rods of chlorine baths, electrolyzers are continuously cooled with water supplied to the casings. The brine, entering the electrolyzer, fills the space between the anode and the body up to the drain hole. During the time determined by the pulsations of the siphon (30-90s), electrolysis proceeds, as a result of which sodium hypochlorite is formed in the solution. The next portion of the brine pushes the brine with the hypochlorite formed as a result of electrolysis out of the electrolysis cells through the drain coarse into the tank under the unit, from where it is fed into the treated water with the help of a dosing device.The technical characteristics of the KG-13 installation are as follows [c.787]
In Sterlitamaksm PA “Caustic”, the excessive consumption of brine is related to its use for the Ji I workshop during the shutdown date D 2, sulfuric acid – by the work of the workshop. low loads, overestimated concentration of waste acid. The overconsumption of hydrochloric acid is explained by the overestimated content of alkali in the return brine, for the group of anodes – by the replacement of electrolysis cells in excess of the POR schedule. [c.33]
Significant savings in the production of chlorine and alkali are achieved with the coordinated action of diaphragm and mercury electrolyzers.The method was developed by the company Diamond Shamro k orp. Only one cleaning is required to prepare the brine feeding this system. Salt brine is purified from calcium and magnesium impurities by treating it with hydroxide and sodium carbonate. The purified brine is adjusted with hydrochloric acid for pH (table salt to a concentration of 318-325 g / l. After that, the brine enters the diaphragm electrolyzer. The electrolyte from the diaphragm electrolyzer enters two evaporators, in which it is successively concentrated to an alkali content of 35 and 50%.In the first stage of evaporation, sodium chloride is precipitated. Part of it goes to saturate the brine that feeds the diaphragm electrolyzer, and the other part – to saturate the depleted brine, leaving it. The Na l content in the electrolyte decreases and an equivalent amount of sodium hydroxide is formed. [c.118]
If pure brine and mercury are used in electrolysis, and platinum serves as the anode, then with a low sodium content in the surface layer of the mercury cathode, i.e.That is, with good mixing, the evolution of hydrogen with an increase in the current density at the cathode will be practically negligible. It would seem that under these conditions it is possible to obtain current outputs close to 100%. However, in practical conditions, this is hindered by the side reduction process at the cathode of chlorine dissolved in the electrolyte. [c.327]
During periodic operation, brine or water is added to the bath and the electrolyte is added with solid salt. [c.378]
During electrolysis, solid salt and water or brine are added to the bath to re-saturate the electrolyte and replenish the water that has been scoured.Therefore, the concentration of sodium chloride by the end of electrolysis does not fall below 125 g l. The electrolyte from the bath is sent to the sump 3 with a steam jacket, where at 80-90 ° for 6-12 hours. particles of graphite are precipitated and all the hypochlorous acid passes into the chloric acid salt. From the settler, the solution is sent to a sand filter 4, and then to a vacuum evaporator 5 with a stirrer, where it is evaporated at 100 ° to beats. weight 1.5. During evaporation, sodium chloride falls out of solution and is separated in suction 6. After washing, sodium chloride is used to prepare the brine.A solution of sodium perchloric acid from the putsch is channeled into crystallization in a cast-iron enamel crystallizer 7. When cooled to 30 °, crystals of chloric acid precipitate. When using pure salt obtained by evaporation of purified brine or by some other method, the brine purification step is simplified. The electrolyte supplied to electrolysis has the following approximate composition: 280 g / l Na l, 40-80 g / l Na lOa, 3-6 g / l Na2 r20r and pH = 6-7.It is passed through a cascade of 4-6 electrolyzers connected in series by electric current and liquid current. As the electrolyte passes through the cascade, the concentration of sodium chloride in it decreases, while the concentration of chlorate increases. [c.65]
Electrolyte concentrated solution of chloride salt (300-330 g Na l or 280-300 g CC in 1 liter), previously purified from calcium salts (adding soda), magnesium (adding alkali), and sometimes and sulfates (with the addition of barium chloride) well-settled and filtered, heated to 35-70 ° C, sometimes slightly alkalized.The electrolyte from the brine preparation section is fed through iron pipelines to the electrolysis shop along a series of electrolysers connected in series and further to the feeder of each electrolyzer. [c.83]
Fresh purified brine (up to 310 g / l Na I) is fed to the upper end of the electrolyzer through a liquid rotameter (which measures the amount of brine supplied to the electrolysis) and a tube attached to the electrolyzer cover. Depleted electrolyte (260-275 g / l Na l) is removed together with chlorine through a rubberized tube passing through the bottom of the electrolyzer at its lower end.To maintain a minimum electrolyte level in the cell, the end of the brine outlet tube is located above the bottom. [c.356]
MANUAL INJECTOR AN 1
What are cookies (biscuits)?
A cookie is an alphanumeric file (text) that is stored on your computer when you visit our site (or updated for repeat visits).
How do we use cookies on our website?
We do not collect or process any information that allows the direct identification of personal data.Cookies are used on this site for statistical purposes (in relation to statistics of visits to our site), as well as in order to ensure the proper functioning of the service, and to provide more content-aware.
Can I disable cookies?
Yes, you can do this at any time by changing your browser settings when using Cookies.
How to disable cookie support in different browsers:
Google Chrome
You have to click on the menu (top right corner), Settings tab> Show advanced settings.In the “Privacy” section, click the Settings button should be happy. The following cookies can be made in “Cookies”:
- Biscuit Removal
- Default block cookies
- Default Allow Cookies
- Saving cookies and data to close the default browser
- Specifying cookie exceptions from specific sites or domains
Internet Explorer
In the browser menu (top right): Tools> Internet Options> Security, click the Sites button.Use the slider to set this level, click OK to confirm the change.
Mozilla Firefox
In the browser menu: Tools> Options> Privacy. Check the Firefox box, “Will use custom settings”.
About cookies (cookies) determines the choice – or not – to Accept Cookies.
Opera
In the browser menu: Tools> Options> Advanced.
About the cookie determines the choice – or not – Cookies.
Safari
Safari from the drop-down menu, select Preferences and click the Security icon. This is where you select the security level in “Accept Cookies”.
Disabling cookies or blocking the service may result in a malfunction of our website. If your browser accepts support for cookies, then you agree to the use of cookies on our website
gaz.wiki – gaz.wiki
Navigation
- Main page
Languages
- Deutsch
- Français
- Nederlands
- Russian
- Italiano
- Español
- Polski
- Português
- Norsk
- Suomen kieli
- Magyar
- Čeština
- Türkçe
- Dansk
- Română
- Svenska
Dorit Manual brine injector HSM – Eurotex
HSM brine injector consists of an IP-45 type impeller pump used for industrial automatic brine brine.The brine pump is equipped with a rubber impeller and is made entirely of high quality stainless steel. Average injection speed from 45 l / min, the pump is directly connected to a flanged electric motor. The electric motor is equipped with a special stainless steel shaft. Fresh brine is filtered through a large filter, then excess brine is collected and returned back to the brine tank. The injection gun can be equipped with single, double or triple needles for muscle or vein injection.Of course, injection guns and needles must be of the same type of high quality steel. The diameter of the needles is 4.5 or 3.00 mm.
Functions and operation:
Double-sided suction filter and re-filtration filter model 10-T-270 are installed on any tanks for water or brine, the diameter of which is not less than 40 cm. The pump is started with a factory-installed switch. After that, the brine can be fed through a hand gun and injected into the meat: injection with single needles for veins or injection with double or triple needles into the muscle.The amount of injected brine is determined by visually checking the weight gain on the scale.
Specifications | HSM-1 | HSM-3 | HSM-5 | HSM-16 | |
Number of single needles | 1 | 3 | 5 | 16 | |
Number of needle heads | 1 | 1 | 1 | 1 | |
Diameter of needles | mm | 4.5 | 4.5 | 4.5 | 3.0 |
Stator voltage | V / Hz | 400/50 | 400/50 | 400/50 | 400/50 |
triggering voltage of non-electrode | Volt | 220 | 220 | 220 | 220 |
Dimensions (packaging) (length x width x height) | cm | 90 x 80 x 55 | 90 x 80 x 55 | 90 x 80 x 55 | 90 x 80 x 55 |
Net weight | kg | 38 | 38 | 38 | 40 |
Gross weight, suitable packaging for sea transportation | kg | 56 | 56 | 56 | 58 |
Light pickle for basic types in python? Ru Python
All I want to do is serialize and unserialize string tuples or ints.
I’ve looked at pickle.dumps () but the byte overhead is significant. In fact, it looks like it takes up about 4x more space than it needs. Also, all I need are basic types and no need to serialize objects.
Marshal is slightly better in terms of space, but the result is full of nasty \ x00 bytes. Ideally, I would like the result to be human-readable.
I was thinking to just use the repr () and eval () functions, but is there an easy way to achieve this without using eval ()?
This is saved in the db, not in the file.Byte is an overhead issue because it can make the difference between requiring a TEXT column versus varchar, and generally the compactness of the data affects all areas of db performance.
Take a look at the json, at least the generated dumps can be read in many other languages.
JSON (JavaScript Object Notation) http://json.org is a subset of JavaScript (ECMA-262 3rd edition) syntax used as a lightweight data interchange format.
I personally will be using yaml. it is on par with json for encoding size, but can be more complex things if needed (like classes, recursive structures).
In [1]: import yaml In [2]: x = [1, 2, 3, 'pants'] In [3]: print (yaml.dump (x)) [1, 2, 3, pants] In [4]: y = yaml.load ('[1, 2, 3, pants]') In [5]: y Out [5]: [1, 2, 3, 'pants'] You may not be using the correct protocol:
>>> import pickle >>> a = range (1, 100) >>> len (pickle.dumps (a)) 492 >>> len (pickle.dumps (a, pickle.HIGHEST_PROTOCOL)) 206 See documentation for pickle data formats.
If you need a space saving solution, you can use Google Protocol Buffers.
Protocol Buffers – Coding
Protocol Buffers – Python Tutorial
The python documentation mentions some built-in persistence functions, but I don’t think any of them are significantly less in the generated files.
You can always use configparser, but there you will only get a string, int, float, bool.
“byte overhead is significant”
Why does it matter? He does the job. If you are running low on disk space, I would be happy to sell you 1TB for $ 500.
Did you launch it? Is performance an issue? Can you demonstrate that serialization performance is an issue?
“I was thinking of just using the repr () and eval () functions, but is there an easy way to accomplish this without using eval ()?”
Nothing easier than roar and eval.
What’s wrong with eval?
Is there “someone could insert malicious code into the file where I serialized my lists”?
Who – specifically – is going to find and edit this file in order to inject malicious code? Anything you do to protect it (ie Encryption) removes the “simple” from it.
Fortunately, there is a solution that uses COMPRESSION and solves a common problem involving any arbitrary Python object, including new classes.Instead of managing small tuples, it is sometimes better to use the DRY tool.
Your code will be clearer and easier to refactor in similar future situations.
Module y_serial.py :: Python Object Stores with SQLite
“Serialization + persistence :: in a few lines of code, compress and annotate Python objects in SQLite, then chronologically rebuild them by non-SQL keywords. Most useful “standard” database module for storing schema-less data. “
http://yserial.sourceforge.net
[If you’re still worried why not insert these tuples into the dictionary and then apply y_serial to the dictionary. Probably any overhead will disappear due to transparent compression in the background by zlib.]
In terms of readability, the documentation also provides details on why cPickle was chosen over json.
.
 R Technology!
R Technology! Learn about our top 10 aquarium fish for 5- to 20-gallon tanks.
Learn about our top 10 aquarium fish for 5- to 20-gallon tanks.
 Art Holds 12 records in Manitoba Bow Hunters Record Club Book
Art Holds 12 records in Manitoba Bow Hunters Record Club Book Trust Xpress Bill Pay to manage your bills.
Trust Xpress Bill Pay to manage your bills. F3 bengal cat for sale
F3 bengal cat for sale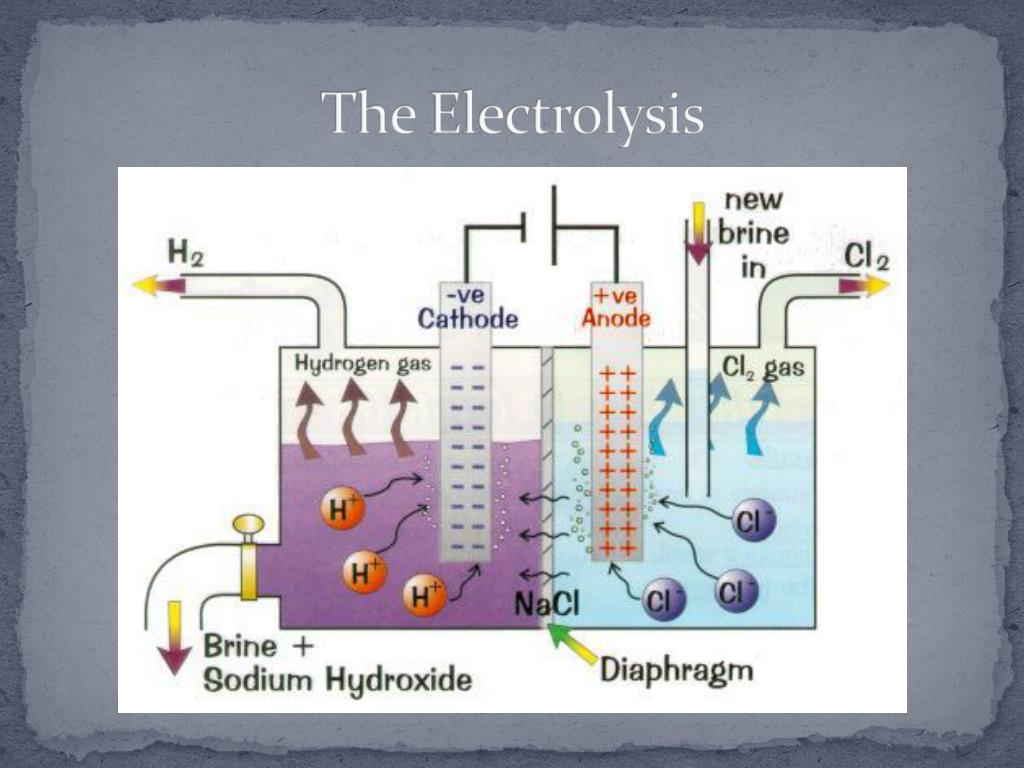 1
1