What are the key components of traditional lacrosse pockets. How does East Coast Dyes contribute to the evolution of lacrosse equipment. What are the advantages of different stringing techniques in men’s lacrosse. How do mesh and traditional pockets compare in performance.
The Evolution of Lacrosse Pockets: From Traditional to Modern Innovations
Lacrosse, a sport with deep Native American roots, has seen significant advancements in equipment technology over the years. One area that has experienced notable evolution is the lacrosse pocket. East Coast Dyes (ECD) has been at the forefront of this innovation, particularly in the realm of traditional pockets and modern hybrids.
Traditional lacrosse pockets, originally crafted from natural materials like leather and gut, have been a staple of the sport for centuries. These pockets offer unique characteristics that many players still prefer today. However, the introduction of synthetic materials and innovative stringing techniques has led to the development of mesh pockets and hybrid designs.

What defines a traditional lacrosse pocket?
A traditional lacrosse pocket is characterized by its use of leather strings and nylon cross-lacing to create a customized channel for ball control. These pockets typically consist of four main leather runners, several nylon cross-laces, and sidewall strings that connect the pocket to the head of the stick.
East Coast Dyes: Revolutionizing Lacrosse Stringing and Materials
East Coast Dyes has made significant contributions to the lacrosse industry by developing innovative products and techniques for both traditional and modern pocket styles. Their approach combines respect for the sport’s heritage with cutting-edge materials science.
How has East Coast Dyes impacted lacrosse equipment?
ECD has introduced several game-changing products, including:
- High-performance mesh materials with enhanced grip and durability
- Advanced dyes and treatments for leather strings
- Specialized tools for precise pocket stringing
- Hybrid pocket designs that blend traditional and mesh elements
These innovations have allowed players to fine-tune their pockets for optimal performance, leading to improved ball control and shooting accuracy.

The Art and Science of Lacrosse Pocket Stringing
Stringing a lacrosse pocket is both an art form and a technical skill. The process requires a deep understanding of how different materials and techniques affect the pocket’s performance.
What are the key elements of pocket stringing?
Effective pocket stringing involves several crucial factors:
- Tension control: Balancing tightness for ball retention and release
- Channel creation: Forming a consistent path for the ball
- Whip adjustment: Fine-tuning the pocket’s release point
- Material selection: Choosing appropriate strings and mesh for desired feel
- Compliance with regulations: Ensuring the pocket meets official standards
East Coast Dyes provides resources and products to help players master these elements, whether they’re working with traditional, mesh, or hybrid pockets.
Mesh vs. Traditional: Analyzing Performance Differences
The debate between mesh and traditional pockets has been ongoing in the lacrosse community for years. Each style offers distinct advantages and drawbacks that can significantly impact a player’s game.

How do mesh and traditional pockets compare?
Mesh pockets are known for their consistency and low maintenance, while traditional pockets offer a more customized feel and potentially greater ball control. Here’s a breakdown of their characteristics:
| Aspect | Mesh Pockets | Traditional Pockets |
|---|---|---|
| Consistency | High | Variable |
| Customization | Moderate | High |
| Maintenance | Low | High |
| Weather Resistance | Good | Fair |
| Break-in Time | Short | Long |
East Coast Dyes offers products for both styles, allowing players to choose based on their preferences and playing conditions.
Hybrid Pockets: The Best of Both Worlds?
Recognizing the strengths of both traditional and mesh pockets, East Coast Dyes has been instrumental in developing hybrid designs that aim to combine the best features of each style.
What advantages do hybrid pockets offer?
Hybrid pockets typically incorporate elements such as:
- Mesh-backed traditional leather runners for improved consistency
- Synthetic materials that mimic the feel of traditional pockets
- Customizable stringing patterns that blend mesh and traditional techniques
- Weather-resistant treatments on natural materials
These innovations allow players to experience the customized feel of a traditional pocket with the added consistency and durability often associated with mesh.

Optimizing Pocket Performance for Different Positions
Different lacrosse positions require varying pocket characteristics to maximize performance. East Coast Dyes recognizes this and offers specialized products and stringing patterns tailored to specific on-field roles.
How do pocket needs differ by position?
Consider the following position-specific requirements:
- Attackmen: Quick release and precise ball control
- Midfielders: Versatile pockets that balance control and quick transitions
- Defensemen: Deeper pockets for secure ball retention during checks
- Goalies: Wide, shallow pockets for rapid ball redistribution
East Coast Dyes provides guidelines and products to help players customize their pockets according to their positional needs, whether using traditional, mesh, or hybrid designs.
The Impact of Regulations on Lacrosse Pocket Design
Lacrosse pocket regulations have a significant influence on the development and use of different stringing techniques and materials. East Coast Dyes works within these parameters to create compliant yet high-performing products.

How do regulations affect pocket design?
Key regulatory considerations include:
- Depth restrictions to prevent excessive hold on the ball
- Shooting string limitations to control release speeds
- Material restrictions for player safety and fair play
- Pocket alignment requirements to maintain consistent performance
East Coast Dyes ensures that their products and stringing guides adhere to official regulations while still allowing for maximum customization within legal limits.
The Future of Lacrosse Pocket Technology
As the sport of lacrosse continues to evolve, so too does the technology behind equipment design. East Coast Dyes remains at the cutting edge of these advancements, constantly researching and developing new materials and techniques.
What innovations can we expect in lacrosse pockets?
Potential future developments may include:
- Smart materials that adapt to playing conditions
- Eco-friendly, sustainable pocket materials
- Advanced customization through 3D printing technology
- Integration of data-tracking sensors for performance analysis
East Coast Dyes is likely to be at the forefront of these innovations, continuing to push the boundaries of what’s possible in lacrosse pocket design.

The world of lacrosse pockets is rich with tradition and ripe for innovation. East Coast Dyes has played a pivotal role in bridging the gap between time-honored techniques and modern advancements. By offering a wide range of products for traditional, mesh, and hybrid pockets, they cater to the diverse needs of players at all levels of the sport.
As the game evolves, so too will the technology behind the equipment. Players, coaches, and enthusiasts alike can look forward to continued improvements in pocket design, stringing techniques, and material science. These advancements will not only enhance performance on the field but also contribute to the growth and accessibility of the sport as a whole.
Whether you’re a traditionalist who swears by leather and cross-lace or a progressive player embracing the latest in mesh technology, East Coast Dyes provides the tools and knowledge to optimize your lacrosse experience. The future of lacrosse pockets is bright, and it’s clear that the blend of tradition and innovation will continue to shape the sport for years to come.

Dyes
Plants have been used for natural dyeing since before recorded history. The staining properties of plants were noted by humans and have been used to obtain and retain these colors from plants throughout history. Native plants and their resultant dyes have been used to enhance people’s lives through decoration of animal skins, fabrics, crafts, hair, and even their bodies.
Types of Dyes
Natural dye materials that produce durable, strong colors and do not require the addition of other substances to obtain the desired outcome are called substantive or direct dyes. Sumac (Rhus spp.) and walnut (Juglans spp.) are native plant examples of direct dyes. Because these species are high in tannic acid, they do not require additional substances to be added for the dye to attach to fibers and form a durable bond. Dyes that need this type of assistance are called adjective or mordant dyes.
Mordants
Mordants are water-soluble chemicals, usually metallic salts, which create a bond between dye and fiber thus increasing the adherence of various dyes to the item being dyed. The actual color one gets from a natural dye depends not only on the source of the dye but also on the mordant, and the item being dyed.
Most mordant recipes also call for the addition of cream of tartar or tartaric acid. Use of this readily available spice is important because it reduces fiber stiffness that can occur because of mordanting. It can also increase brightness.
| Mordant | Effect |
|---|---|
| Alum | Brightens the colors obtained from a dye source |
| Iron/Coppers | Darkens/saddens hues, produces blacks, brown, gray |
| Copper vitriol | Improves likelihood of obtaining a green hue |
| Tin | Produces bright colors especially yellows, oranges, reds |
| Chrome | Highly toxic – should not be used for dyeing at home |
Plants Used for Dyes
Throughout the world, evidence of natural dyeing in many ancient cultures has been discovered.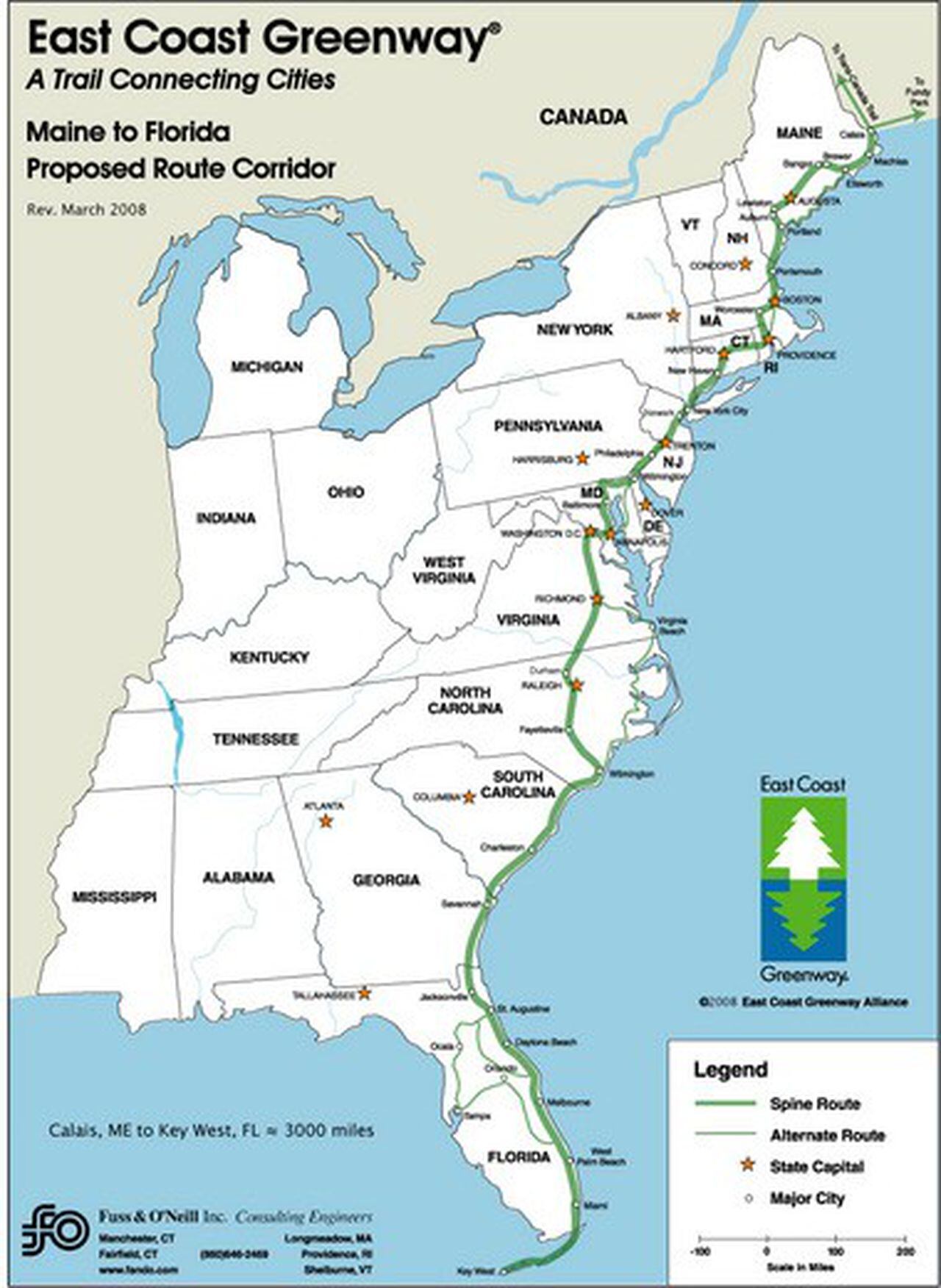 Textile fragments dyed red from roots of an old world species of madder (Rubia tinctoria) have been found in Pakistan, dating around 2500 BC. Similar dyed fabrics were found in the tombs of Egypt.
Textile fragments dyed red from roots of an old world species of madder (Rubia tinctoria) have been found in Pakistan, dating around 2500 BC. Similar dyed fabrics were found in the tombs of Egypt.
Finely woven Hopi wicker plaques made from rabbitbrush and sumac stems colored with native and commercial dyes. Photo by Teresa Prendusi.
Mordants can be used to increase color intensity such as in this Southwestern–style rug. Photo by Teresa Prendusi.
- Tyrean purple dye was discovered in 1500 B.C. and was produced from the glandular secretions of a number of mollusk species.
- This purple dye was extremely expensive to produce as it required nearly 12,000 mollusks to produce 3.5 ounces of dye.
- Tyrean purple became the color of royalty.
- Lichens were used to produce ochril, a purple dye, which was called the “poor person’s purple”.
Native North American Plants Used for Dyes
European settlers in North America learned from Native Americans to use native plants to produce various colored dyes (see Table 2).
| PLANT | Number of Uses |
|---|---|
| Mountain Alder | 53 |
| Red Alder | 21 |
| Bloodroot | 20 |
| Rubber rabbitbrush | 16 |
| Smooth sumac | 16 |
| Canaigre dock | 14 |
| Eastern cottonwood | 13 |
| Black walnut | 12 |
| Skunkbrush sumac | 11 |
| Butternut | 9 |
Bloodroot (Sanguinaria canadensis). Photo by Dave Moore.
Photo by Dave Moore.
Bloodroot (
Sanguinaria canadensis)
Bloodroot (Sanguinaria canadensis) was used to produce red dyes. Green dyes were made from algae and yellow dyes were made from lichens. Early colonists discovered that colors produced by the Native Americans quickly faded, thus suggesting that mordants may not have been used.
Mountain alder (Alnus incana).
Mountain alder (
Alnus incana)
This small, riparian tree has been used by many native tribes to make a brown, red-brown, or orange-red dye to darken hides, stain bark used in basketry and dye porcupine quills. Inner bark was used to make yellow dye. Outer bark was used to make a flaming red hair dye. Some tribes mixed this species with grindstone dust or black earth to make a black dye. Bark was used to wash and restore the brown color to old moccasins.
In the western United States, various layers of red alder bark, Alnus rubra, yield red, red-brown, brown, orange, and yellow dyes. These colors have been used to stain baskets, hides, moccasins, hair, quills, fishnets, canoes, cloth, and other items.
These colors have been used to stain baskets, hides, moccasins, hair, quills, fishnets, canoes, cloth, and other items.
Smooth sumac (Rhus glabra), an important dye plant, with fall colors.
Smooth sumac (
Rhus glabra)
This deciduous shrub is a widely distributed throughout most of the contiguous United States. It is readily recognized by its thicket-forming habit, milky sap, compound leaves, and dense, terminal panicles of bright red drupes. A variety of dye colors can be obtained from different parts of the plant depending on the mordant used.
The leaves are rich in tannin and can be used as a direct dye. Leaves can be collected as they fall in the autumn and used as a brown dye. The twigs and root are also rich in tannin. A black and a red dye can be obtained from the fruit. A black dye is obtained from the leaves, bark, and roots. An orange or yellow dye is obtained from the roots harvested in spring. A light yellow dye is obtained from the pulp of the stems.
Butternut (Juglans cinerea)
Butternut (
Juglans cinerea)
This tree native to the eastern United States was important as a food and dye source. Native Americans used the bark to make a brown dye and young roots to make a black dye. Using an iron mordant, brown dye can be changed to a charcoal or gray color.
- The famous gray coats that the Confederate Army wore during the Civil War were colored with dye made from butternuts.
- Confederate soldiers were called “butternuts” because of their dyed uniforms.
Rubus
The genus Rubus belongs to the rose family. Common names include raspberry, blackberry, blackcap, and thimbleberry. Varieties of blackberry include dewberry, boysenberry, and loganberry. This group consists of erect, arching or trailing, deciduous and evergreen shrubs found wild in Europe, North America, and Asia.
These berries are actually aggregate fruits, which means they are composed of individual drupelets, held together by almost invisible hairs. Some berry canes may be armed with formidable spines and make great security hedges, while others may be nearly spineless. All parts of the blackberry plant (berries, leaves, canes) yield dye colors.
Some berry canes may be armed with formidable spines and make great security hedges, while others may be nearly spineless. All parts of the blackberry plant (berries, leaves, canes) yield dye colors.
Rubus species are important for food, medicine, and dyes. Photo by Marry Ellen (Mel) Harte © Forestryimages.org.
| Dye Color | Plant Common Name (Additional Colors) |
|---|---|
| Yellow Dyes | Yarrow (green, black) |
| Honey Locust | |
| Golden wild-indigo (green) | |
| Tall cinquefoil (black, green, orange, red) | |
| Pecan (brown) | |
| Indiangrass (brown, green) | |
| Orange Dyes | Western comandra (brown, yellow) |
| Prairie Bluets (brown, yellow) | |
| Bloodroot (brown, yellow) | |
| Sassafras (black, green, purple, yellow) | |
| Eastern Cottonwood (black, brown, yellow) | |
| Plains Coreopsis (black, green, yellow, brown) | |
| Red Dyes | Ozark chinkapin (black, yellow, brown) |
| Sumac (yellow, green, brown, black) | |
| Chokecherry | |
| Prairie Parsley (yellow, brown) | |
| Slippery Elm (brown, green, yellow) | |
| Black Willow (black, green, orange, yellow) | |
| Purple / Blue Dyes | Indian blanket (black, green, yellow) |
| Hairy coneflower (brown, green, yellow, black) | |
| Red Mulberry (brown, yellow, green) | |
| Mountain alder (brown, red, orange) | |
| Summer Grape (orange, yellow, black) | |
| Black Locust (black, green, yellow, brown) | |
| Green Dyes | Butterfly milkweed (yellow) |
| Texas Paintbrush (green, red, yellow) | |
| Basket flower (yellow) | |
| Sagebrush (yellow, gray) | |
| Stinging nettle | |
| Goldenrod (yellow, brown) | |
| Gray Dyes | Iris (black) |
| Butternut (brown) | |
| Canaigre Dock (yellow, green, brown) | |
| Brown Dyes | Prickly poppy (green, orange, yellow) |
| Texas Paintbrush (green, red, yellow) | |
| Elderberry (yellow) | |
| Downy Phlox (brown, green, yellow) | |
| Black Dyes | Northern Catalpa (brown, yellow) |
| Sumac (yellow, red, green, brown) | |
| May-apple (brown, yellow) | |
| Sand Evening Primrose (green, orange, red, yellow) |
Did You Know?
- The tissues of canaigre dock (Rumex hymenosepalus) – a southwest desert native plant used to make yellow, gray or green dye, and widely noted for its medicinal, edible, and social uses – contain toxic oxalate.
 The needlelike crystals produce pain and edema when touched by lips, tongue or skin.
The needlelike crystals produce pain and edema when touched by lips, tongue or skin. - Eastern cottonwood used to make a variety of dyes was a sign to early pioneers that they were near water. Ribbons of cottonwoods were found across the prairie where underground watercourses were located.
- Prior to chemical synthesis of indigo dye, blue jeans and cotton were dyed with a blue dye derived from tropical indigo bush, native to India. Mayo indigo, from the Sonoran desert was used for blue dye for thousands of years.
- Rubber rabbitbrush, a western native, can be used to create both green and yellow dyes. The bark produces green dye while flowers produce yellow dye.
- Not only is stinging nettle edible, it can be used to create a green dye. Stinging nettle can cause severe skin irritation, but is useful for dyes, fiber, and food.
For More Information
- Native American Ethnobotany Database – explore more about native plants used for natural dyes.

- Making Natural Dyes from Plants – examine some readily available natural plant dyes.
Canaigre dock (Rumex hymenosepalus). Photo by Teresa Prendusi.
Stinging nettle (Urtica dioica).
Traditional Uses, Chemical Constituents, and Biological Activities of Bixa orellana L.: A Review
1. Perecin MB, Bovi OA, Maia NB. Pesquisa com plantas aromáticas, medicinais corantes: o papel do Instituto Agronômico. O Agronômico. 2002;54:21–24. [Google Scholar]
2. Elias MEA, Schroth G, Macêdo JLV, Mota MSS, D’Angelo SA. Mineral nutrition, growth and yields of annatto trees (Bixa orellana) in agroforestry on an Amazonian Ferralsol. Experimental Agriculture. 2002;38(3):277–289. [Google Scholar]
3. Bastos ARR, Carvalho JG, Assis RP, Filho ABC. Marcha de absorção de nutrientes em urucum ( Bixa orellana L.) tipo cultivado piave vermelha em fase de viveiro. Cerne. 1999;5:76–85. [Google Scholar]
1999;5:76–85. [Google Scholar]
4. Thomas EP, Colvin M, Rosen SB, Zuccarini C, Petzer S. Buffalo, NY, USA: Medical Research Council; 2005. HIV prevalence study and costing analysis undertaken for the development of an HIV/AIDS workplace strategy for buffalo city municipality. [Google Scholar]
5. Franco CF. O Agronegócio do urucum na região Nordeste. 1 Reunião Nacional da Cadeia Produtiva de Urucum, CD-Rom, 2007.
6. Giorgi A, De Marinis P, Granelli G, Chiesa LM, Panseri S. Secondary metabolite profile, antioxidant capacity, and mosquito repellent activity of Bixa orellana from Brazilian Amazon region. Journal of Chemistry. 2013;2013:10 pages.409826 [Google Scholar]
7. Silva JS, Moura MD, Oliveira RAG, Diniz MFF, Barbosa-Filho JM. Natural product inhibitors of ovarian neoplasia. Phytomedicine. 2003;10(2-3):221–232. [PubMed] [Google Scholar]
8. Sousa FCF, Melo CTV, Citó COM, et al. Plantas medicinais e seus constituintes bioativos: uma revisão da bioatividade e potenciais benefícios nos distúrbios da ansiedade em modelos animais. Revista Brasileira de Farmacognosia. 2008;18:642–654. [Google Scholar]
Revista Brasileira de Farmacognosia. 2008;18:642–654. [Google Scholar]
9. Quintans LJ, Jr., Almeida JRGS, Lima JT, et al. Plants with anticonvulsant properties—a review. Revista Brasileira de Farmacognosia. 2008;18:798–819. [Google Scholar]
10. Falcão HDS, Leite JA, Barbosa-Filho JM, et al. Gastric and duodenal antiulcer activity of alkaloids: a review. Molecules. 2008;13(12):3198–3223. [PMC free article] [PubMed] [Google Scholar]
11. Falcão HS, Mariath IR, Diniz MFFM, Batista LM, Barbosa-Filho JM. Plants of the American continent with antiulcer activity. Phytomedicine. 2008;15(1-2):132–146. [PubMed] [Google Scholar]
12. Mariath IR, Falcão HDS, Barbosa-Filho JM, et al. Plants of the American continent with antimalarial activity. Revista Brasileira de Farmacognosia. 2009;19(1):158–192. [Google Scholar]
13. Hon JER, Soares PM, Melo CL, et al. Atividade farmacológica da monocrotalina isolada de plantas do gênero Crotalaria
. Revista Brasileira de Farmacognosia. 2010;20:453–458. [Google Scholar]
Revista Brasileira de Farmacognosia. 2010;20:453–458. [Google Scholar]
14. Almeida RN, Navarro DS, Barbosa-Filho JM. Plants with central analgesic activity. Phytomedicine. 2001;8(4):310–322. [PubMed] [Google Scholar]
15. Jesus NZT, Falcão HS, Gomes IF, et al. Tannins, peptic ulcers and related mechanisms. International Journal of Molecular Sciences. 2012;13(3):3203–3228. [PMC free article] [PubMed] [Google Scholar]
16. Rocha LG, Almeida JRGS, Macêdo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12(6-7):514–535. [PubMed] [Google Scholar]
17. Lima GRM, Montenegro CA, Almeida CLF, Athayde-Filho PF, Barbosa-Filho JM, Batista LM. Database survey of anti-inflammatory plants in South America: a review. International Journal of Molecular Sciences. 2011;12(4):2692–2749. [PMC free article] [PubMed] [Google Scholar]
18. De Lira Mota KS, Dias GEN, Pinto MEF, et al. Flavonoids with gastroprotective activity. Molecules. 2009;14(3):979–1012. [PMC free article] [PubMed] [Google Scholar]
Molecules. 2009;14(3):979–1012. [PMC free article] [PubMed] [Google Scholar]
19. Souto AL, Tavares JF, da Silva MS, de Diniz MFFM, de Athayde-Filho PF, Barbosa Filho JM. Anti-inflammatory activity of alkaloids: an update from 2000 to 2010. Molecules. 2011;16(10):8515–8534. [PMC free article] [PubMed] [Google Scholar]
20. Silva FL, Fischer DCH, Tavares JF, Silva MS, de Athayde-Filho PF, Barbosa-Filho JM. Compilation of secondary metabolites from Bidens pilosa L. Molecules. 2011;16(2):1070–1102. [PMC free article] [PubMed] [Google Scholar]
21. Filho JR, de Sousa Falcão H, Batista LM, Filho JMB, Piuvezam MR. Effects of plant extracts on HIV-1 protease. Current HIV Research. 2010;8(7):531–544. [PubMed] [Google Scholar]
22. Barbosa-Filho JM, Alencar AA, Nunes XP, et al. Sources of alpha-, beta-, gamma-, delta- and epsilon-carotenes: a twentieth century review. Revista Brasileirta de Farmacogn. 2008;18(1):135–154. [Google Scholar]
23. Silva SNS, Amaral CLF, Rebouças TNH. Adoption of conservation practices on farm and selection of varieties by producers of annatto in the city of Vitoria da Conquista-BA. Revista Brasileira de Agroecologica. 2010;5:106–113. [Google Scholar]
Silva SNS, Amaral CLF, Rebouças TNH. Adoption of conservation practices on farm and selection of varieties by producers of annatto in the city of Vitoria da Conquista-BA. Revista Brasileira de Agroecologica. 2010;5:106–113. [Google Scholar]
24. Bandoni AL, Mendiondo ME, Rondina RVD, Coussio JD. Survey of Argentine medicinal plants. I. Folklore and phytochemical screening. Lloydia. 1972;35(1–4):69–80. [PubMed] [Google Scholar]
25. Garcia GH, Campos R, de Torres RA, et al. Antiherpetic activity of some Argentine medicinal plants. Fitoterapia. 1990;61(6):542–546. [Google Scholar]
26. Brandao MGL, Grandi TSM, Rocha EMM, Sawyer DR, Krettli AU. Survey of medicinal plants used as antimalarials in the Amazon. Journal of Ethnopharmacology. 1992;36(2):175–182. [PubMed] [Google Scholar]
27. Corrêa MP. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas. Vol. 4. Rio de Janeiro, Brasil: Ministério da Agricultura/IBDF; 1978. [Google Scholar]
[Google Scholar]
28. Hirschmann GS, de Arias AR. A survey of medicinal plants of minas gerais, Brazil. Journal of Ethnopharmacology. 1990;29(2):159–172. [PubMed] [Google Scholar]
29. Otero R, Fonnegra R, Jiménez SL, et al. Snakebites and ethnobotany in the northwest region of Colombia. Part I: traditional use of plants. Journal of Ethnopharmacology. 2000;71(3):493–504. [PubMed] [Google Scholar]
30. Garcia-Barriga H. Flora Medicinal de Colombia. Vol. 2. Bogota, Colombia: Universidad Nacional; 1975. [Google Scholar]
31. Roig y Mesa JT. Plantas Medicinales, Aromaticas o Venenosas de Cuba. Havana, Cuba: Ministério de Agricultura; 1945. [Google Scholar]
32. Cáceres A, Menéndez H, Méndez E, et al. Antigonorrhoeal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. Journal of Ethnopharmacology. 1995;48(2):85–88. [PubMed] [Google Scholar]
33. Duke JA, Martinez RV. Amazonian Ethnobotanical Dictionary. Boca Raton, Fla, USA: CRC Press; 1994. [Google Scholar]
Boca Raton, Fla, USA: CRC Press; 1994. [Google Scholar]
34. Ramirez VR, Mostacero LJ, Garcia AE, et al. Vegetales Empleados en Medicina Tradicional Norperuana. Trujillo, Peru: Banco Agrario del Peru, Universidad Nacional de Trujillo; 1988. [Google Scholar]
35. Villar R, Calleja JM, Morales C, Caceres A. Screening of 17 Guatemalan medicinal plants for platelet antiaggregant activity. Phytotherapy Research. 1997;11:441–445. [Google Scholar]
36. Giron LM, Freire V, Alonzo A, Caceres A. Ethnobotanical survey of the medicinal flora used by the Caribs of Guatemala. Journal of Ethnopharmacology. 1991;34(2-3):173–187. [PubMed] [Google Scholar]
37. Lentz DL. Medicinal and other economic plants of the Paya of Honduras. Economic Botany. 1993;47(4):358–370. [Google Scholar]
38. Lentz DL, Clark AM, Hufford CD, et al. Antimicrobial properties of Honduran medicinal plants. Journal of Ethnopharmacology. 1998;63(3):253–263. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
39. Morrison EY, West ME. The effect of Bixa orellana (Annatto) on blood sugar levels in the anaesthetized dog. West Indian Medical Journal. 1985;34(1):38–42. [PubMed] [Google Scholar]
40. Coe FG, Anderson GJ. Screening of medicinal plants used by the Garífuna of eastern Nicaragua for bioactive compounds. Journal of Ethnopharmacology. 1996;53(1):29–50. [PubMed] [Google Scholar]
41. Barrett B. Medicinal plants of Nicaragua’s Atlantic Coast. Economic Botany. 1994;48(1):8–20. [Google Scholar]
42. Schmeda-Hirschmann G, de Arias AR. A screening method for natural products on triatomine bugs. Phytotherapy Research. 1992;6(2):68–73. [Google Scholar]
43. Simpson GE. Folk medicine in Trinidad. The Journal of American Folklore. 1962;75(298):326–340. [Google Scholar]
44. Mahabir D, Gulliford MC. Use of medicinal plants for diabetes in Trinidad and Tobago. Revista Panamericana de Salud Publica. 1997;1(3):174–179. [PubMed] [Google Scholar]
1997;1(3):174–179. [PubMed] [Google Scholar]
45. Ayensu ES. Medicinal plants of the West Indies. Unpublished Manuscript, 1978. [Google Scholar]
46. Mercadante AZ, Steck A, Rodriguez-Amaya D, Pfander H, Britton G. Isolation of methyl 9′Z-APO-6′-lycopenoate from Bixa Orellana
. Phytochemistry. 1996;41(4):1201–1203. [Google Scholar]
47. Angelucci E, Arima HK, Kumagai EA, Annatto I. Preliminary data of the chemical composition. Coletanea do Instituto de Tecnologia de Alimentos. 1980;11:89–96. [Google Scholar]
48. Tirimanna ASL. Study of the carotenoid pigments of Bixa orellana L. Seeds by thin layer chromatography. Mikrochimica Acta. 1981;76(1-2):11–16. [Google Scholar]
49. Barbosa-Filho JM, Silva-Filho RN, Lira BF, et al. Teor de bixina em quatro variedades de Bixa orellana L. cultivadas na Paraíba. Revista Brasileira de Farmacognosia. 1998;7-8:41–47. [Google Scholar]
50. Mercadante AZ, Steck A, Pfander H. Isolation and structure elucidation of minor carotenoids from annatto (Bixa orellana L.) seeds. Phytochemistry. 1997;46(8):1379–1383. [Google Scholar]
Isolation and structure elucidation of minor carotenoids from annatto (Bixa orellana L.) seeds. Phytochemistry. 1997;46(8):1379–1383. [Google Scholar]
51. Prentice-Hernandez C, Rusig O. Annatto (Bixa orellana L.) extract obtained using ethyl alcohol as a solvent. Arquivos de Biologia e Tecnologia. 1992;35:63–74. [Google Scholar]
52. Mercadante AZ. Composition of carotenoids from annatto. ACS Symposium Series. 2001;775:92–101. [Google Scholar]
53. Scotter MJ, Wilson LA, Appleton GP, Castle L. Analysis of Annatto (Bixa orellana) Food Coloring Formulations. 1. Determination of Coloring Components and Colored Thermal Degradation Products by High-Performance Liquid Chromatography with Photodiode Array Detection. Journal of Agricultural and Food Chemistry. 1998;46(3):1031–1038. [Google Scholar]
54. Degnan AJ, von Elbe JH, Hartel RW. Extraction of annatto seed pigment by supercritical carbon dioxide. Journal of Food Science. 1991;56:1655–1659. [Google Scholar]
1991;56:1655–1659. [Google Scholar]
55. Mercadante AZ, Steck A, Pfander H. Isolation and identification of new apocarotenoids from annatto (Bixa orellana) seeds. Journal of Agricultural and Food Chemistry. 1997;45(4):1050–1054. [Google Scholar]
56. Anderson SG, Nair MG, Chandra A, Morrison E. Supercritical fluid carbon dioxide extraction of annatto seeds and quantification of trans-bixin by high pressure liquid chromatography. Phytochemical Analysis. 1997;8:247–249. [Google Scholar]
57. Barbosa-Filho JM. Bixa orellana: Retrospectiva de usos populares, atividades biológicas, fitoquímica e emprego na fitocosmética, no continente americano. João Pessoa, Brazil: Simpósio Brasileiro do Urucum—SIMBRAU; 2006. [Google Scholar]
58. Revilla J. Plantas da Amazônia: Oportunidades Econômicas e Sustentáveis. 2nd edition. Manaus: Programa de Desenvolvimento Empresarial e Tecnológico; 2001. [Google Scholar]
59. Oliveira F, Akisue G, Akisue MK. Farmacognosia. São Paulo, Brazil: Atheneu; 1996. [Google Scholar]
Farmacognosia. São Paulo, Brazil: Atheneu; 1996. [Google Scholar]
60. Alonso J. Tratado de Fitofármacos y Nutracêuticos. Rosário, Argentina: Corpus; 2004. [Google Scholar]
61. Franco CO, Fabri EG, Barreiro Neto M, Manfiolli MH, Harder MNC, Rucker NC. Urucum: Sistema de Produção para o Brasil. João Pessoa, Brazil: Emepa-Pb, Apta; 2008. [Google Scholar]
62. Venugopalan PA, Giridhar GA, Ravishankar AG. Food, Ethanobotanical and diversified applications of Bixa orellana L.: a scope for its improvement through biotechnological mediation. Indian Journal of Fundamental and Applied Life Sciences. 2011;1:9–31. [Google Scholar]
63. Taylor L. Herbal Secrets of the Rainforest. 2nd edition. Rocklin, Calif, USA: Sage Press; 2002. [Google Scholar]
64. Oliveira JS. Caracterização, Extração e Purificação por Cromatografia de Compostos de Urucum (Bixa orellana L.) [tesis doctoral en Ingeniería Químic] Florianópolis, Brazil: Universidade Federal de Santa Catarina; 2005. [Google Scholar]
[Google Scholar]
65. Mercadante AZ, Steck A, Pfander H. Three minor carotenoids from annatto (Bixa orellana) seeds. Phytochemistry. 1999;52(1):135–139. [Google Scholar]
66. Galindo-Cuspinera V, Lubran MB, Rankin SA. Comparison of volatile compounds in water- and oil-soluble annatto (Bixa orellana L.) extracts. Journal of Agricultural and Food Chemistry. 2002;50(7):2010–2015. [PubMed] [Google Scholar]
67. Alves de Lima RO, Azevedo L, Ribeiro LR, Salvadori DMF. Study on the mutagenicity and antimutagenicity of a natural food colour (annatto) in mouse bone marrow cells. Food and Chemical Toxicology. 2003;41(2):189–192. [PubMed] [Google Scholar]
68. Lima LRP, Oliveira TT, Nagem TJ, et al. Bixina, norbixina e quercetina e seus efeitos no metabolismo lipídico de coelhos. Brazilian Journal of Veterinary Research and Animal Science. 2001;38:196–200. [Google Scholar]
69. Fontana JD, Mendes SV, Persike DS, Peracetta LF, Passos M. Carotenóides: cores atraentes e ação biológica. Biotecnologia Ciência & Desenvolvimento. 2000;2:p. 13. [Google Scholar]
Carotenóides: cores atraentes e ação biológica. Biotecnologia Ciência & Desenvolvimento. 2000;2:p. 13. [Google Scholar]
70. Penna CA, Radice M, Gutkind GO, et al. Antibacterial and antifungal activities of some Argentinean plants. Fitoterapia. 1994;65(2):172–174. [Google Scholar]
71. Broussalis AM, Ferraro GE, Martino VS, Pinzón R, Coussio JD, Alvarez JC. Argentine plants as potential source of insecticidal compounds. Journal of Ethnopharmacology. 1999;67(2):219–223. [PubMed] [Google Scholar]
72. Rojas de Arias A, Schmeda-Hirschmann G, Falcao A. Feeding deterrency and insecticidal effects of plant extracts on Lutzomyia longipalpis. Phytotherapy Research. 1992;6(2):64–67. [Google Scholar]
73. Pinheiro MS, Rouquayrol MZ. Molluscicidal activity of plants from Northeast Brazil. Revista Brasileira de Pesquisas Médicas e Biológicas. 1974;7:389–394. [PubMed] [Google Scholar]
74. Hagiwara A, Imai N, Ichihara T, et al. A thirteen-week oral toxicity study of annatto extract (norbixin), a natural food color extracted from the seed coat of annatto (Bixa orellana L.), in Sprague-Dawley rats. Food and Chemical Toxicology. 2003;41(8):1157–1164. [PubMed] [Google Scholar]
A thirteen-week oral toxicity study of annatto extract (norbixin), a natural food color extracted from the seed coat of annatto (Bixa orellana L.), in Sprague-Dawley rats. Food and Chemical Toxicology. 2003;41(8):1157–1164. [PubMed] [Google Scholar]
75. Almeida CR, Silva RB, Marques MJ, Chavasco JK. Evaluation of antiparasitic activity of hydroethanolic extracts from root, stem and leaf of Bixa orellana L. on Leishmania amazonensis samples. Revista da Universidade Vale do Rio Verde. 2012;10(2):384–391. [Google Scholar]
76. Lopes MV, Desoti VC, Caleare ADO, Ueda-Nakamura T, Silva SO, Nakamura CV. Mitochondria superoxide anion production contributes to geranylgeraniol-induced death in leishmania amazonensis. Evidence-Based Complementary and Alternative Medicine. 2012;2012:9 pages.298320 [PMC free article] [PubMed] [Google Scholar]
77. Ferreira JM, Sousa DF, Dantas MB, et al. Effects of Bixa orellana L. seeds on hyperlipidemia. Phytotherapy Research. 2013;27(1):144–147. [PubMed] [Google Scholar]
Phytotherapy Research. 2013;27(1):144–147. [PubMed] [Google Scholar]
78. Benoit PS, Fong HHS, Svoboda GH, Farnsworth NR. Biological and phytochemical evaluation of plants. XIV. Antiinflammatory evaluation of 163 species of plants. Lloydia. 1976;39(2-3):160–171. [PubMed] [Google Scholar]
79. Carabajal D, Casaco A, Arruzazabala L, Gonzalez R, Fuentes V. Pharmacological screening of plant decoctions commonly used in Cuban folk medicine. Journal of Ethnopharmacology. 1991;33(1-2):21–24. [PubMed] [Google Scholar]
80. Valdés AFC, Martínez JM, Rodríguez DA, Lizama RS, Gaitén YG. Actividad antimalárica de un extracto hidroalcohólico de Bixa orellanaL. Revista Cubana de Medicina Tropical. 2011;63:181–185. [PubMed] [Google Scholar]
81. García AD, Sánchez HR, Lizama RS. Citotoxicidad de extractos de plantas medicinales sobre la línea celular de carcinoma de pulmón humano A549. Revista Cubana de Farmácia. 2011;45:101–108. [Google Scholar]
[Google Scholar]
82. Freixa B, Vila R, Vargas L, Lozano N, Adzet T, Canigueral S. Screening for antifungal activity of nineteen Latin American plants. Phytotherapy Research. 1998;12(6):427–430. [Google Scholar]
83. Cáceres A, López B, González S, Berger I, Tada I, Maki J. Plants used in Guatemala for the treatment of protozoal infections. I. Screening of activity to bacteria, fungi and American trypanosomes of 13 native plants. Journal of Ethnopharmacology. 1998;62(3):195–202. [PubMed] [Google Scholar]
84. Caceres A, Cano O, Samayoa B, Aguilar L. Plants used in Guatemala for the treatment of gastrointestinal disorders. 1. Screening of 84 plants against enterobacteria. Journal of Ethnopharmacology. 1990;30(1):55–73. [PubMed] [Google Scholar]
85. Matsui AS, Hoskin S, Kashiwagi M, et al. A survey of natural products from Hawaii and other areas of the Pacific for an antifertility effect in mice. Internationale Zeitschrift fur Klinische Pharmakologie, Therapie, und Toxikologie. 1971;5(1):65–69. [PubMed] [Google Scholar]
1971;5(1):65–69. [PubMed] [Google Scholar]
86. Abayomi M, Adebayo AS, Bennett D, Porter R, Campbell JS. In vitro antioxidant activity of Bixa orellana (Annatto) seed extract. Journal of Applied Pharmaceutical Science. 2014;4(2):101–106. [Google Scholar]
87. Dunham NW, Allard KR. A preliminary pharmacologic investigation of the roots of Bixa orellana
. Journal of the American Pharmacist Associantion. 1960;49:218–219. [PubMed] [Google Scholar]
88. Medina FR, Woodbury R. Terrestrial plants molluscicidal to lymnaeid hosts of Fasciliasis hepatica in Puerto Rico. Journal of Agricultura of the University of Puerto Rico. 1979;63:366–376. [Google Scholar]
89. Weniger B, Jiang Y, Oulad-Ali A, Italiano L, Beck JP, Anton R. Biological effects of bixin and Bixa orellana extracts on lymphoid cells in culture. Planta Medica. 1993;59(7, article A680) [Google Scholar]
90. Chariandy CM, Seaforth CE, Phelps RH, Pollard GV, Khambay BPS.
 The needlelike crystals produce pain and edema when touched by lips, tongue or skin.
The needlelike crystals produce pain and edema when touched by lips, tongue or skin.