How durable are diamonds really. Can diamonds actually burn or be destroyed. What temperature causes diamonds to burn. How to properly care for and clean diamond jewelry.
The Myth of Diamond Indestructibility
Diamonds have long been marketed as eternal and indestructible, epitomized by the famous slogan “A Diamond is Forever”. While diamonds are indeed extremely durable, they are not impervious to damage or destruction. This article explores the true nature of diamond durability and provides essential guidance on diamond care and cleaning.
Understanding Diamond Durability: Hardness, Toughness, and Stability
Diamond durability encompasses three key properties:
- Hardness: Resistance to scratching and abrasion
- Toughness: Ability to withstand breaking and chipping
- Stability: Resistance to chemicals and temperature changes
Diamonds excel in hardness, ranking 10 on the Mohs scale – the highest of any natural substance. This means only another diamond can scratch a diamond. However, hardness does not equate to indestructibility.

The Limitations of Diamond Toughness
Despite their hardness, diamonds can break if struck with sufficient force in the right spot. Diamonds have cleavage planes – directions where atomic bonds are weaker. A skilled diamond cutter can exploit these planes to split a diamond, but this vulnerability also means diamonds can chip or crack if impacted along these lines.
Can Diamonds Really Burn?
Contrary to popular belief, diamonds can indeed burn under certain conditions. At what temperature do diamonds burn? Diamonds will combust at approximately 1562°F (850°C). This temperature can be reached in house fires or by jewelers’ torches, potentially causing irreversible damage to the stone.
The Science Behind Diamond Combustion
When exposed to high temperatures in the presence of oxygen, the carbon atoms in a diamond will react to form carbon dioxide gas. This process can cause a diamond to literally vanish, leaving behind no ash – only the released CO2.
Diamond Stability: Resistance to Chemicals and Temperature Changes
Diamonds exhibit remarkable stability in most conditions. They resist virtually all acids and can withstand the heat generated during the cutting process. However, diamonds are vulnerable to thermal shock – sudden, extreme temperature changes that can create new fractures or exacerbate existing ones.

Protecting Diamonds from Thermal Damage
To prevent thermal shock:
- Avoid exposing diamond jewelry to rapid temperature fluctuations
- Remove diamond rings before activities involving extreme heat, like cooking or metalworking
- Store diamonds separately from other jewelry to prevent temperature transfer
Essential Diamond Care and Cleaning Techniques
Proper care and cleaning are crucial for maintaining the beauty and integrity of diamond jewelry. What are the safest methods for cleaning diamonds at home?
Safe Cleaning Methods for Diamonds
- Use a soft, lint-free cloth to gently wipe the diamond
- Soak in warm water mixed with mild dish soap
- Brush gently with a soft-bristled toothbrush to remove dirt
- Rinse thoroughly with clean water
- Pat dry with a clean, soft cloth
Professional Cleaning and Inspection
While regular at-home cleaning is beneficial, professional cleaning and inspection by a jeweler are recommended at least once a year. This ensures thorough cleaning and allows for early detection of any potential issues with the setting or stone.

Avoiding Harsh Cleaning Methods
Certain cleaning methods can potentially damage diamonds or their settings. Which cleaning techniques should be avoided for diamond jewelry?
- Ultrasonic cleaners: Can loosen stones in their settings
- Steam cleaners: May cause thermal shock
- Abrasive household cleaners: Can scratch metal settings
- Chlorine and harsh chemicals: May damage some diamond treatments
These methods are best left to professional jewelers who can assess the jewelry’s condition and use appropriate techniques safely.
Protecting Diamond Jewelry in Daily Life
While diamonds are highly durable, they are not immune to wear and tear from daily activities. How can you protect your diamond jewelry during everyday wear?
Tips for Preserving Diamond Jewelry
- Remove diamond rings before engaging in sports or manual labor
- Store diamond jewelry separately to prevent scratching other pieces
- Take off diamond jewelry before applying lotions, perfumes, or cosmetics
- Have prongs and settings checked regularly by a jeweler
- Consider insuring valuable diamond pieces against loss or damage
The Impact of Diamond Cut on Durability
The way a diamond is cut can affect its durability. Certain cutting styles may be more prone to chipping or damage. How does diamond cut influence a stone’s vulnerability?

Vulnerable Diamond Cuts
Cuts with pointed corners or ends are generally more susceptible to damage. These include:
- Marquise cut
- Pear cut
- Princess cut
These shapes often require protective settings, such as V-shaped prongs, to shield vulnerable points from impacts.
Durable Diamond Cuts
Cuts with rounded edges tend to be more resistant to chipping. Examples include:
- Round brilliant cut
- Oval cut
- Cushion cut
These shapes distribute force more evenly, reducing the risk of damage from accidental impacts.
Understanding Diamond Treatments and Their Impact on Care
Some diamonds undergo treatments to enhance their appearance or durability. How do these treatments affect diamond care requirements?
Common Diamond Treatments
- Fracture filling: Improves clarity but may be vulnerable to heat
- Laser drilling: Creates tiny channels to remove inclusions
- HPHT color enhancement: Uses high pressure and temperature to alter color
Treated diamonds may require special care. For instance, fracture-filled diamonds should not be cleaned with ultrasonic or steam cleaners, as the heat can damage the filling material.

Caring for Treated Diamonds
If you own a treated diamond:
- Inform your jeweler about the treatment before any repair work
- Avoid exposing the diamond to extreme heat
- Use only gentle cleaning methods at home
- Have the diamond inspected regularly by a professional
The Role of Diamond Settings in Protection
A diamond’s setting plays a crucial role in protecting the stone from damage. How do different setting styles impact a diamond’s durability?
Protective Setting Styles
- Bezel settings: Surround the diamond’s girdle, offering excellent protection
- Channel settings: Secure diamonds between metal walls, shielding edges
- Tension settings: Use the metal’s tension to hold the diamond securely
Potentially Vulnerable Settings
- Prong settings: Can snag on clothing or objects if prongs become loose
- Illusion settings: May expose diamond edges to potential damage
- Floating or minimalist settings: Offer less overall protection to the stone
Regular inspection of your diamond’s setting is crucial, regardless of style. Loose prongs or worn metal can compromise the diamond’s security and increase the risk of loss or damage.

Debunking Common Diamond Care Myths
Misinformation about diamond care abounds. What are some common misconceptions about diamond durability and maintenance?
Myth: Diamonds are Completely Indestructible
While extremely hard, diamonds can chip, crack, or even burn under certain conditions. Proper care is essential to maintain their beauty and integrity.
Myth: Diamonds Never Need Cleaning
Diamonds accumulate oils, dirt, and grime over time, which can dull their sparkle. Regular cleaning is necessary to maintain their brilliance.
Myth: All Cleaning Methods are Safe for Diamonds
Harsh chemicals and abrasive cleaning methods can damage diamonds or their settings. Stick to gentle, approved cleaning techniques or seek professional cleaning.
Myth: Diamond Hardness Means They Can’t Scratch Other Jewelry
Diamonds can easily scratch softer gemstones and metals. Store diamond jewelry separately to prevent damage to other pieces.
The Environmental Impact of Diamond Care Products
As consumers become more environmentally conscious, it’s important to consider the ecological impact of diamond care practices. How can diamond owners maintain their jewelry responsibly?
Eco-Friendly Diamond Cleaning Solutions
- Use biodegradable, phosphate-free dish soap for cleaning
- Opt for microfiber cloths instead of disposable wipes
- Choose cleaning brushes with natural bristles
Sustainable Diamond Care Practices
Adopting sustainable care practices not only benefits the environment but can also extend the life of your diamond jewelry:
- Regularly inspect and maintain settings to prevent stone loss
- Choose durable settings that reduce the need for frequent repairs
- Consider recycled or ethically sourced materials for replacement settings
The Future of Diamond Durability: Synthetic vs. Natural Diamonds
As lab-grown diamonds become more prevalent in the market, questions arise about their durability compared to natural diamonds. Are there differences in care requirements between synthetic and natural diamonds?
Comparing Synthetic and Natural Diamond Durability
Synthetic diamonds have the same chemical composition and physical properties as natural diamonds, including:

- Identical hardness (10 on the Mohs scale)
- Similar toughness and cleavage planes
- Equivalent resistance to chemicals
This means that synthetic diamonds require the same care and cleaning procedures as their natural counterparts.
Advancements in Diamond Technology
Ongoing research in diamond synthesis may lead to innovations in diamond durability:
- Development of diamonds with fewer inclusions, potentially increasing overall toughness
- Creation of diamonds with modified crystal structures to enhance specific properties
- Exploration of new diamond treatments to improve stability and resistance to extreme conditions
While these advancements are exciting, it’s important to note that they are still in experimental stages and not widely available in the consumer market.
Professional Diamond Care: When to Seek Expert Help
While regular at-home care is essential, certain situations call for professional intervention. When should you consult a jeweler for diamond care?
Signs Your Diamond Needs Professional Attention
- Visible chips, cracks, or abrasions on the diamond’s surface
- Loose or damaged prongs in the setting
- Significant loss of brilliance that doesn’t improve with cleaning
- Discoloration or clouding within the diamond
Professional Diamond Care Services
Expert jewelers can provide specialized care for your diamonds, including:

- Deep cleaning using professional-grade equipment
- Repairing or replacing damaged settings
- Re-polishing to remove surface scratches
- Assessing and addressing any structural issues
Regular professional inspections, typically recommended annually, can help catch and address potential problems before they become severe.
Insuring Your Diamond: Protection Beyond Physical Care
While proper care can prevent many issues, accidents can still happen. How can insurance provide an additional layer of protection for your diamond jewelry?
Types of Diamond Insurance
- Homeowner’s or renter’s insurance riders
- Specialized jewelry insurance policies
- Travel insurance for valuable items
Factors to Consider When Insuring Diamonds
When selecting diamond insurance, consider:
- Coverage limits and deductibles
- Replacement vs. cash payout options
- Coverage for loss, theft, and damage
- Requirements for regular appraisals
Proper documentation, including recent appraisals and photographs, can streamline the insurance process in case of loss or damage.
Ethical Considerations in Diamond Care and Maintenance
As consumers become more aware of ethical issues in the diamond industry, how can these concerns be integrated into diamond care practices?
Sustainable Diamond Care Products
Choose cleaning products and tools that align with ethical and environmental values:
- Eco-friendly, biodegradable cleaning solutions
- Reusable cleaning cloths and brushes
- Packaging made from recycled or sustainable materials
Ethical Repair and Replacement Options
When repairs or replacements are necessary, consider:
- Jewelers who source ethically mined or lab-grown diamonds
- Recycled precious metals for settings
- Local artisans who prioritize sustainable practices
By incorporating these ethical considerations into your diamond care routine, you can maintain your jewelry while supporting responsible industry practices.
Diamond Care and Cleaning Guide
Consumers know diamond from the slogan “A Diamond is Forever”. With some care considerations this is relatively true. The Asscher cut was popular decades ago and is again a very popular cut style. This stone has exceptional quality being D color and VVS2 Clarity.
“A Diamond is Forever” is one of the world’s best-known advertising slogans. It has many different meanings. It refers to diamond’s timeless appeal. It refers to diamond’s icy beauty. And it also refers to diamond’s durability. One result of the diamond formation process is its incredible durability.
Durability is a gemstone’s ability to withstand wear, heat, and chemicals. Durability consists of three properties: hardness, toughness, and stability. Hardness means how well a gemstone resists scratches and abrasion. Toughness describes how well a gemstone resists breaking and chipping. Stability means how well a diamond resists chemicals and temperature changes.
Toughness describes how well a gemstone resists breaking and chipping. Stability means how well a diamond resists chemicals and temperature changes.
Hardness
Gem and mineral hardness is measured on the Mohs scale. The scale originated in 1812 when German mineralogist Friedrich Mohs chose ten minerals and assigned numbers to them, based on the relative ease or difficulty with which one could be scratched by another. But the Mohs scale is deceptive. The steps between the minerals are not evenly spaced. For example, diamond is only one number away, but it’s many times harder than gems in the corundum family. Only a diamond can scratch a diamond.
Something the Mohs scale doesn’t show, but that’s equally important to the diamond industry, is that diamond can also scratch any of the precious metals used for settings. That means a diamond that’s loose in its setting can wear through a prong over time.
Diamond rates highest on the Mohs hardness scale, at number 10.:max_bytes(150000):strip_icc()/steam-burns-overview-4507433_color2-5c6f2661c9e77c000149e479.png)
Toughness
Any stone, including a diamond, will break if it’s hit hard enough in the right place. Toughness is a measure of how well a gem can survive an impact and resist breaking, chipping, or cracking.
Diamonds are tougher in the directions where the atoms are bonded tightly together, less tough where they’re not so tightly bonded.
Cutting styles with pointed corners or ends are often set with prongs to protect the corners from chipping. – Courtesy Ambar Diamonds
The weakest directions are the ones where the atoms are farthest apart. It’s easier to break a diamond in those directions, which are called cleavage directions. A cutter can cleave a diamond by hitting it sharply in the cleavage direction. But even after cutting, a hard blow can still cleave a diamond. This can happen during the setting process, or even when it’s being worn.
Stability
Stability is a term that describes how well a diamond resists temperature changes and chemicals. Diamonds are very stable. They’re invulnerable to virtually all acids, for one thing. The cutting process generates a lot of heat, but diamonds usually endure intact. Situations that are more threatening to a diamond’s stability are those that involve sudden and extreme temperature changes. Those changes can cause thermal shock and create new fractures and cleavages or cause existing ones to spread.
Diamonds are very stable. They’re invulnerable to virtually all acids, for one thing. The cutting process generates a lot of heat, but diamonds usually endure intact. Situations that are more threatening to a diamond’s stability are those that involve sudden and extreme temperature changes. Those changes can cause thermal shock and create new fractures and cleavages or cause existing ones to spread.
Diamonds will burn at about 1562°F (850°C). House fires and jewelers’ torches can reach that temperature.
A house fire caused the white, cloudy appearance of this diamond (left). The stone was recut to remove the burned area, reducing the diamond’s size, but leaving no sign that it was ever damaged (right).
Cleaning
Diamonds can be cleaned safely with lint-free cloths, commercial jewelry cleaning solutions, and household detergents.
Harsher cleaning methods are not recommended for home use. These include powdered abrasive household cleansers, ultrasonic cleaners, and steam cleaners.
Ultrasonic cleaners and steam cleaners can loosen gemstones in their settings. Jewelry professionals carefully examine jewelry for loose stones before using these devices.
Can Diamonds Actually Burn? – International Gem Society
Question: I came across this statement and was wondering if anyone can verify it:
A diamond is the hardest natural substance on earth, but if it is placed in an oven and the temperature is raised to about 763º Celsius (1405º Fahrenheit), it will simply vanish, without even ash remaining. Only a little carbon dioxide will have been released.
Is this true? Can diamonds burn up and simply vanish?
Diamonds can occur in rocks like kimberlite, which form as magma cools. Photo by James St. John. Licensed under CC By 2.0.
Answer: Although diamonds originate deep underground and form under extreme temperatures, diamonds can indeed burn under certain conditions.
Diamonds Burn Like Anything Carbon
As pure crystalline carbon (C), diamonds have the exact same chemistry as graphite (though they have different molecular structures). If strongly heated in the presence of oxygen (air), carbon will react with the oxygen (burn) to form carbon dioxide gas (CO2). Other compounds containing carbon, such as plant material or flesh, will decompose quickly when heated strongly. At normal temperatures and in the presence of moisture and bacteria, they will decompose very slowly into various gases, including methane (CH4) and carbon dioxide.
We all finish up as carbon dioxide and dust eventually. Recycling, anyone?
Cheers,
John Burgess, Mapua Nelson, NZ
Impure Diamonds Burn and Leave Ashes
Yes, diamonds burn. There are many substantiated insurance claims of diamonds being destroyed in fires. As far as I know, the bit about no ash remaining is theoretical. Being pure carbon, the combustion of diamond does produce CO2. But just how many absolutely pure diamonds exist? Any color in diamonds is produced by non-carbon impurities. In addition to the oxygen, which takes up residence on the surface of a diamond (attaching itself almost automatically to the free molecular bonding sites), most diamonds are at least partially nitrogenated.
Being pure carbon, the combustion of diamond does produce CO2. But just how many absolutely pure diamonds exist? Any color in diamonds is produced by non-carbon impurities. In addition to the oxygen, which takes up residence on the surface of a diamond (attaching itself almost automatically to the free molecular bonding sites), most diamonds are at least partially nitrogenated.
So, technically, if you have a non-nitrogenated, flawless, pure-white diamond, you can turn it into CO2 with the application of heat. But who would want to?
Boy, isn’t a CRC Handbook of Chemistry and Physics a handy thing to have around?
D.R.
How to Make Diamonds Vanish
Diamonds burn, but the temperature at which they burn depends on whether or not the diamonds are in contact with air. The temperature of diamond ignition in pure oxygen is 690º C to 840º C.
In a stream of oxygen gas, diamonds burn initially at a low red heat. They will gradually rise in temperature and reach a white heat. Then, the diamonds will burn uninterruptedly with a pale-blue flame, even after the removal of the oxygen heat source. The diamond crystals will gradually decrease in size and finally disappear. The flame at the last moment will flicker brightly and then disappear, leaving not a trace of ash or residue.
They will gradually rise in temperature and reach a white heat. Then, the diamonds will burn uninterruptedly with a pale-blue flame, even after the removal of the oxygen heat source. The diamond crystals will gradually decrease in size and finally disappear. The flame at the last moment will flicker brightly and then disappear, leaving not a trace of ash or residue.
For this to take place in an air mixture, the heat must remain applied directly on the diamonds at all times. If removed, the diamonds won’t continue to burn, because oxygen diluted with nitrogen won’t support combustion.
Ron Campbell, Central Coast Gem Lab
Measuring a diamond, photo by Mauro Cateb. Licensed under CC By 2.0.
Are diamonds really forever? | New-Science.ru
Actually, chemically – no. Graphite is a more stable form of carbon. So diamond will turn into graphite. But that is not all.
The origin of the word diamond comes from the Greek word Ἀδάμας, which means “irresistible”.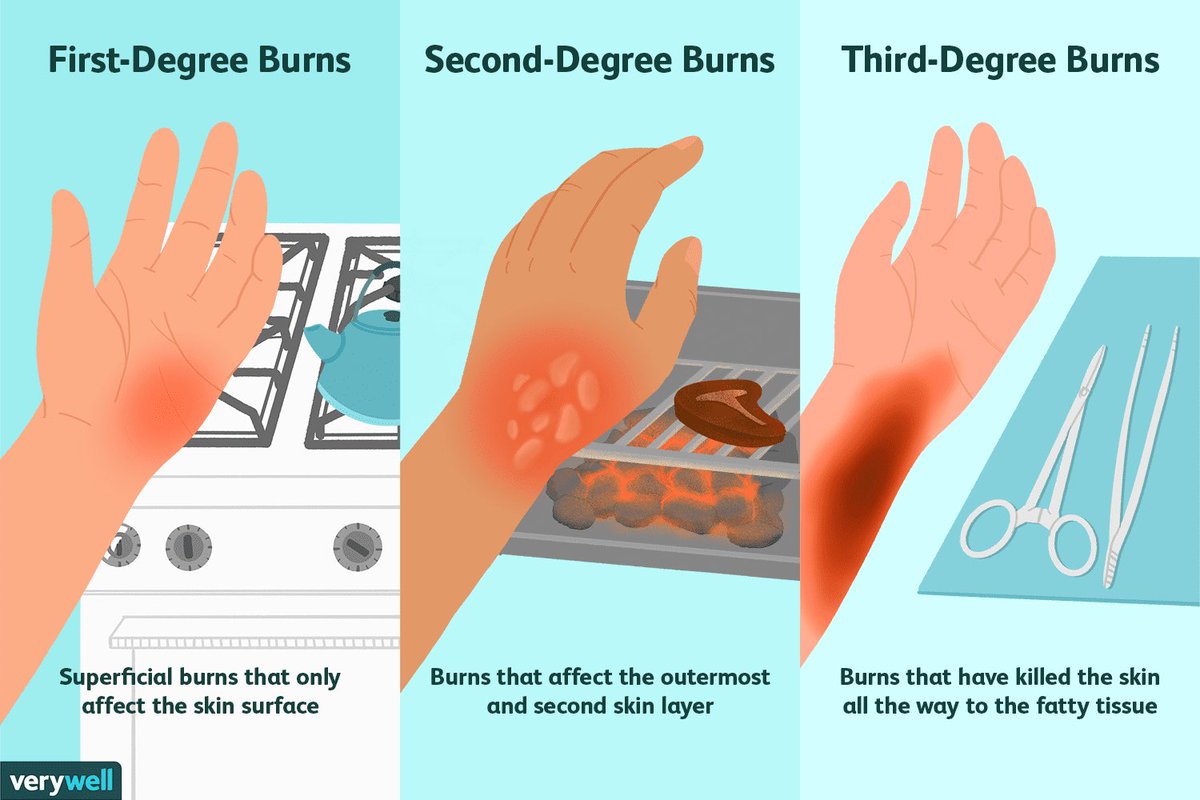 First discovered sometime in the 4th century BC, some of the world’s most valuable diamonds date back 100 million to billions of years ago.
First discovered sometime in the 4th century BC, some of the world’s most valuable diamonds date back 100 million to billions of years ago.
Yes, diamonds are not forever. Instead, they can turn into the coolest everyday object – graphite. Yes, the same graphite that is in your pencil.
Or diamonds can simply burn to carbon dioxide.
So why can a diamond turn into graphite or burn? And how long can this last?
Diamond is an allotrope of carbon
Carbon itself is carbon. An element with atomic number 6 and a non-metal.
But when carbon bonds with other carbon atoms, it can create many structures, each with a unique set of properties. These different forms are called allotropes.
Allotropes are a feature of non-metallic elements such as carbon, silicon and phosphorus (which has as many as 6 allotropes).
Carbon has many allotropes. This is due to its valency. Carbon has four available electrons that it can share with other elements to create compounds. This valency gives it a unique flexibility to form different structures when combined with other carbons.
This valency gives it a unique flexibility to form different structures when combined with other carbons.
Diamond has an octahedral structure in which each individual carbon atom attaches to four other carbon atoms to form a kind of three-sided pyramidal structure.
Notice how the topmost carbon bonds in the diamond look like a three sided pyramid.
Other carbon allotropes form sheets (graphite and graphene), spheres (buckminsterfullerene) and even some strange nanostructures.
Graphite, not diamond, is the most stable allotrope of carbon.
Although diamond’s tetrahedral structure makes it the hardest substance known to mankind, it is not the most stable form of carbon.
This title belongs to graphite.
You see, diamond is a metastable structure of carbon. Metastable in chemistry means that the structure is more or less stable under certain conditions, but there is an even more stable state.
A common analogy is to imagine a ball rolling down a valley. The most stable place for the ball will be the bottom of the valley (as seen from the deeper valley in the picture below).
The most stable place for the ball will be the bottom of the valley (as seen from the deeper valley in the picture below).
Now imagine that the ball is stuck in a smaller hole (smaller valley where the ball can be seen).
The ball is stable in the smaller well, but since the well is higher than the valley floor, this is not the most stable state. But the ball will remain there unless work is done to get the ball out of the well and down to the bottom of the valley.
Understanding the stability of chemicals.
Our diamond is like a ball in a well, and graphite is like a ball at the foot of a hill.
Chemically speaking, diamond is kinetically stable because it is trapped in a well, whereas it is thermodynamically unstable because there is a more stable form of graphite that it can turn into under the right conditions.
So why don’t diamonds turn into graphite?
So, if there is a more stable form, why didn’t all diamonds turn into graphite? For two reasons.
First, the diamond is stable in the conditions that exist on Earth. In addition, graphite is only a few electron volts more stable than diamond (on Earth). The difference in the stability of diamond and graphite on Earth is not so great.
Second, it takes a lot of energy to convert diamond to graphite.
In other words, the energy required to bring a diamond out of the well to the bottom of the valley, where it turns into graphite, is very high.
Chemists and geologists tried to turn diamond into graphite. They found that when a diamond is compressed with an indenter (basically a sharp object that can pierce the diamond), the surface of the diamond in contact with the indenter turns into graphite.
If squeezing a diamond isn’t your style, scientists have also found that low pressure and very high temperatures (1500 to 1900 degrees Celsius or more) will work. Add iron to the mixture and this will speed up the process (called graphitisation).
Diamonds also have their weaknesses
However, do not subject diamonds to high pressures if you want to turn it into graphite. Diamonds are more stable at high pressure than graphite, which is why they form in the Earth’s mantle (and even occasionally on asteroids). However, under certain conditions, diamonds can turn into graphite even under high pressure.
Diamonds are more stable at high pressure than graphite, which is why they form in the Earth’s mantle (and even occasionally on asteroids). However, under certain conditions, diamonds can turn into graphite even under high pressure.
How long will diamonds last?
With that said, a diamond on your engagement ring or crown of the Queen of England will most likely last forever.
But if you use your diamond as a tool for cutting or grinding things, especially things made of iron, then you might want to look into it.
The part of the diamond that comes into contact with iron (or whatever the diamond cuts) can get hot enough to turn into graphite. If even tiny bits of diamond turn into graphite every time you cut something, the diamond will eventually turn completely into graphite.
Or you can simply burn a diamond with just a magnifying glass and the sun. This is exactly what they did in 1694 years, two people, naturalist Giuseppe Averani and physician Cipriano Targioni from Florence. They took a magnifying glass and shone sunlight on the diamond, and the stone disappeared.
They took a magnifying glass and shone sunlight on the diamond, and the stone disappeared.
The question is whether a diamond burns in fire. Can a diamond be melted? A stone with an ancient history
Diamond is a precious stone, but its properties were appreciated by physicists only in the 16th century. And this despite the fact that the stone was found several centuries earlier. Of course, in order to appreciate the full significance of the mineral, it took a lot of experiments. They gave information about the hardness of the stone, the melting point of the diamond, and other physical characteristics. But since then, the stone has been used not only as a beautiful accessory, but also for industrial purposes.
The evaluation was carried out in special laboratories. And as a result, the chemical composition of diamond, the structure of its crystal lattice, and several phenomena were discovered.
Melting of diamond
Experiments related to the melting temperature
As is known, the crystal lattice of a substance has the shape of a tetrahedron with covalent bonds between carbon atoms. It is possible that this structure was the reason for several discoveries related to the melting of diamond.
It is possible that this structure was the reason for several discoveries related to the melting of diamond.
Encyclopedias of minerals give indicators of diamond melting of 3700-4000 degrees Celsius. But this is not entirely accurate information, since they do not lend themselves to generally accepted patterns. In particular, the following effects were found during melting:
- Using high temperatures (2000 degrees Celsius without oxygen), diamond can be turned into graphite. At the same time, the further behavior of this substance with increasing temperature defies logical explanation. But the process cannot be reversed. In extreme cases, you can get a synthetic stone, the crystal lattice of which will differ from natural diamonds.
- If the stone is heated to a temperature of 850-1000 degrees Celsius, it turns into carbon dioxide, that is, it disappears without a trace. Such an experiment was carried out in 1694 by researchers from Italy Targioni and Averani, trying to melt the stones and combine them into one diamond.

- Research was also carried out in 2010 in California, where a group of physicists concluded that it was impossible to achieve diamond melting if the temperature of the stone was gradually increased. To find out the melting index, it is necessary, in addition to temperature, to influence the diamond with pressure, and this makes measurement difficult. To really convert the diamond into a liquid state, scientists needed to make a lot of effort. To do this, they used laser pulses that acted on the stone for several nanoseconds. At the same time, the stone in liquid form was obtained at a pressure 40 million times higher than atmospheric pressure at sea level. In addition, if the pressure dropped to 11 million atmospheres, and the temperature on the surface of the mineral was 50 thousand Kelvin, then hard pieces appeared on the stone. They did not sink in the rest of the liquid and outwardly resembled pieces of ice. With a further decrease in the pressure indicator, the pieces accumulated, forming “icebergs” afloat.
 Scientists have compared that this is how carbon behaves in the composition of the planets Neptune and Uranus, on the surface of these celestial bodies there are also oceans with liquid diamond. But in order to prove this assumption, it is necessary to send satellites to the planets, which at the moment cannot be quickly implemented.
Scientists have compared that this is how carbon behaves in the composition of the planets Neptune and Uranus, on the surface of these celestial bodies there are also oceans with liquid diamond. But in order to prove this assumption, it is necessary to send satellites to the planets, which at the moment cannot be quickly implemented. - If you act on a stone with short light pulses in the ultraviolet range, then small depressions will appear in the mineral. Thus, the experiment confirms the disappearance of the stone under the action of powerful ultraviolet, that is, the transformation of diamond into carbon dioxide. Therefore, diamond-based ultraviolet lasers quickly break down and become unusable. But don’t worry about the fact that the diamond on the jewelry will disappear over time: to remove one microgram of the mineral, you have to keep the diamond under ultraviolet light for about 10 billion years.
So, the melting index is an interesting characteristic of a diamond. It is still a subject for study. With the advent of technology, scientists are finding new ways to test this characteristic. Based on it, one can draw conclusions about the origin of the stone, discover new ways to use diamond.
It is still a subject for study. With the advent of technology, scientists are finding new ways to test this characteristic. Based on it, one can draw conclusions about the origin of the stone, discover new ways to use diamond.
This state, located on the border of crystalline and molten forms, will not only help to better understand the structure and characteristics of diamond, but will also reveal the secrets of distant planets.
“Diamonds can be called a chemical compound familiar to the Earth. However, in order to melt it, it is not enough just high temperature – extremely high pressure is also necessary, which, in turn, makes it difficult to regulate heating,” says one of the authors of the study Hermann Eggert.
Scientists once succeeded in melting a diamond, but during that experiment, the scientific team could not properly regulate the process and measure the parameters. It can be said that the result of that experiment was accidental.
Diamonds are an exceptionally strong material, and this alone makes melting them a formidable task. But, in addition, there is another feature that makes the process almost impossible. The fact is that when the temperature rises, diamonds do not want to retain their nature and change their physical properties, turning into graphite. And already this compound turns into a liquid. The scientists had to go to the trick – to bring the diamond to the point where it begins to turn into graphite, and keep it in it.
But, in addition, there is another feature that makes the process almost impossible. The fact is that when the temperature rises, diamonds do not want to retain their nature and change their physical properties, turning into graphite. And already this compound turns into a liquid. The scientists had to go to the trick – to bring the diamond to the point where it begins to turn into graphite, and keep it in it.
The gas giants Uranus and Neptune are one of the few places in the universe known to us where ultra-high temperatures combine with super-high pressure. To replicate these natural conditions, Eggert and his colleagues placed a ten-carat, half-millimeter-thick natural diamond into a laser machine that can generate enormous pressure.
At a pressure 40 million times higher than the pressure on Earth at sea level, the diamond turned into a liquid substance. After that, the scientists began to gradually reduce the pressure and temperature in the installation. At around 11 million times the normal pressure on Earth and a temperature of about 50,000 degrees Kelvin, solid fragments began to form in the diamond liquid. Empirically, it was possible to establish that the process of their formation is gaining momentum with a decrease in pressure while maintaining the temperature at a constant level.
Empirically, it was possible to establish that the process of their formation is gaining momentum with a decrease in pressure while maintaining the temperature at a constant level.
The further behavior of the sample amazed the scientists. Diamond chips did not stick together, but floated in a liquid medium, just as icebergs float on the expanses of the oceans.
Most materials are less dense in liquid form than in solid form. Water is considered the only exception, since the density of ice is always less than the density of liquid water. Molten diamond exhibits the same qualities.
Analysis shows that Neptune and Uranus are ten percent carbon. Therefore, according to Eggert, the existence of diamond seas on these planets is quite possible. Moreover, such formations would fit perfectly into the theory, since they can explain one of the most interesting mysteries of these gas giants.
On Earth, the magnetic poles almost coincide with the geographic poles. And on Uranus and Neptune, the axis of the magnetic field is sharply shifted from the axis of rotation – the difference is about 60 degrees. The existence of a diamond ocean, which is capable of reflecting and refracting magnetic waves, could well explain such a phenomenon.
The existence of a diamond ocean, which is capable of reflecting and refracting magnetic waves, could well explain such a phenomenon.
Ilya Torbaev, doctor of geological and mineralogical sciences, spoke about the diamond seas and diamond shores of Uranus and Neptune.
“From a physical point of view, the proposed model has no obvious flaws. Yes, we are used to the fact that diamond is a unique mineral for the Earth. But this uniqueness is due only to the lack of sufficient conditions on our planet for the formation of such chemical compounds.
Uranus and Neptune, on the contrary, seem to be created for the synthesis of such substances. High carbon content, extreme pressure and high temperature could have made it so that diamond became as common there as like silicon on Earth. While the physico-chemical component of Eggart’s experiment is beyond doubt, the astronomical part requires verification and proof. But they will have to wait – the next expeditions to Uranus and Neptune are planned only for 2025-2030. ”
”
The word “diamond” comes from Greek. It is translated into Russian as “”. Indeed, to damage this stone, you need to make superhuman efforts. It cuts and scratches all minerals known to us, while itself remains unscathed. Acid does not harm him. Once, out of curiosity, an experiment was carried out in a forge: a diamond was placed on an anvil and hit with a hammer. The iron almost split in two, but the stone remained intact.
Diamond glows with a beautiful bluish color.
Diamond has the highest thermal conductivity of all solids. It is resistant to friction, even against metal. It is the most elastic mineral with the lowest compression ratio. An interesting property of a diamond is to luminesce even under the influence of artificial rays. It glows with all the colors of rainbows and refracts color in an interesting way. This stone seems to be saturated with solar color, and then radiates it. As you know, a natural diamond is ugly, the cut gives it true beauty. A gem made from a cut diamond is called a diamond.
A gem made from a cut diamond is called a diamond.
History of experiments
In the 17th century in England, Boyle managed to burn a diamond by shining a sunbeam on it through a lens. However, in France, the experiment with calcining diamonds in a melting vessel did not give any results. The French jeweler who conducted the experiment found only a thin layer of dark plaque on the stones. At the end of the 17th century, the Italian scientists Averani and Targioni, when trying to fuse two diamonds together, were able to establish the temperature at which a diamond burns – from 720 to 1000 ° C.
Diamond does not melt due to the strong structure of the crystal lattice. All attempts to melt the mineral ended in burning it.
The great French physicist Antoine Lavoisier went further, deciding to place the diamonds in an airtight vessel made of glass and fill it with oxygen. With the help of a large lens, he heated the stones, and they completely burned out. After examining the composition of the air environment, they found that in addition to oxygen, it contains carbon dioxide, which is a combination of oxygen and carbon. Thus, the answer was received: diamonds burn, but only when oxygen is available, i.e. on open air. Burning, the diamond turns into carbon dioxide. That is why, unlike coal, even ash does not remain after the combustion of diamond. The experiments of scientists confirmed another property of diamond: in the absence of oxygen, the diamond does not burn, but its molecular structure changes. At a temperature of 2000 ° C, graphite can be obtained in just 15-30 minutes.
After examining the composition of the air environment, they found that in addition to oxygen, it contains carbon dioxide, which is a combination of oxygen and carbon. Thus, the answer was received: diamonds burn, but only when oxygen is available, i.e. on open air. Burning, the diamond turns into carbon dioxide. That is why, unlike coal, even ash does not remain after the combustion of diamond. The experiments of scientists confirmed another property of diamond: in the absence of oxygen, the diamond does not burn, but its molecular structure changes. At a temperature of 2000 ° C, graphite can be obtained in just 15-30 minutes.
Everything in this world is not eternal. Almost everything eventually turns to dust. And unfortunately no one can change that. Yet there are things in our world that, according to many, are unchanging. Today I want to talk about one such object – a diamond. Diamond is considered to be one of the hardest minerals in the world. But still…
Do you know that diamonds can burn? This fascinating phenomenon was discovered as a result of experiments that were carried out with this mineral. As a result of the experiments, it turned out that at high temperatures (850-1000 degrees C), a very hard mineral changes its structure and turns into the purest carbon dioxide, leaving no other substances. This was first proven in 1694, at the moment when scientists from Italy K.A. Tarjoni and J. Averani tried to combine several small diamonds into one large diamond. Burning temperature at which diamond burns
As a result of the experiments, it turned out that at high temperatures (850-1000 degrees C), a very hard mineral changes its structure and turns into the purest carbon dioxide, leaving no other substances. This was first proven in 1694, at the moment when scientists from Italy K.A. Tarjoni and J. Averani tried to combine several small diamonds into one large diamond. Burning temperature at which diamond burns
in a stream of pure oxygen is a little less: 720-800 degrees C. Moreover, the mineral burns with a beautiful and blue flame.
Again, interesting, in my opinion, is the fact that it is possible to produce ordinary graphite from diamond. To do this, you just need to heat the stone, in the absence of oxygen, to a temperature of 2000 degrees C.
All of the above facts have been proven many times by scientists in practice, and subsequently scientifically substantiated.
So that women remember that diamond is lit
, a diamond on your finger can turn into ordinary graphite from high temperatures. Remember this and be careful, do not get excited.
Remember this and be careful, do not get excited.
Burning diamonds. Video.
Interesting pages of our site:
Bad weather. Interesting facts about rain
Underground boat. Secret developments
Bogomolov accelerator. Is it possible to completely destroy a single country?
And the boiling of the diamond? Does a mineral exist in molten form in the natural environment? We will look for answers to these and other questions in the presented material.
How did diamonds form in the bowels of the Earth?
According to scientists, diamonds could have appeared during the formation of the planet’s core as a result of the impact on the molten magma of enormous pressure. To the surface areas of the earth’s crust, precious stones have advanced due to the processes of gas formation in deep rocks. As a result, the so-called diamond pipes were formed, which are voids in stony soil with large mineral deposits.
Material properties
Before we figure out what the melting point of diamond is, let’s look at the properties of the mineral:
- Diamonds have the highest hardness of any existing fossil.
 For this reason, no material is able to destroy or scratch its surface. He himself can damage any physical object.
For this reason, no material is able to destroy or scratch its surface. He himself can damage any physical object. - Diamond is a highly effective insulator. It is resistant to acids and other aggressive chemical environments.
- Diamond has the highest thermal conductivity of all solid minerals. The gemstone can be held in the palm of your hand as long as you like. At the same time, its temperature will remain unchanged.
- Diamond has a unique luminescence. Light rays of any origin, when passing through a mineral, make it glow brightly and shimmer with all the colors of the rainbow.
Structure
Basically, a diamond is made up of carbon atoms. However, each of them is located in the central part of the tetrahedron – a polyhedron that is formed from the four planes of a triangle. Thus, an extremely strong bond of atoms is ensured. This explains the highest hardness, as well as the impressive melting point of diamond.
Conditions for the melting of diamonds
In 2010, during experiments in the physics laboratory of the University of California at Berkeley, they determined the level of temperature exposure to a diamond, which leads to its melting. Scientists have found that it is impossible to convert the material into a liquid form under normal conditions, regardless of the level of heating. This goal can be achieved only by exposing the diamond not only to temperature, but also to the highest pressure. It is necessary to increase the pressure so that the mineral does not turn into graphite. Thus, the transition of diamond into a liquid form is an extremely difficult process.
Scientists have found that it is impossible to convert the material into a liquid form under normal conditions, regardless of the level of heating. This goal can be achieved only by exposing the diamond not only to temperature, but also to the highest pressure. It is necessary to increase the pressure so that the mineral does not turn into graphite. Thus, the transition of diamond into a liquid form is an extremely difficult process.
What is the melting point and boiling point of diamond?
According to data obtained during the study of the properties of the material, its melting in air under high pressure occurs when heated to 850-1000 ° C. Diamond can be brought to a boil by exposing it to a temperature of 1800 to 2000 ° C in a vacuum. In both cases, upon cooling, the mineral is converted into graphite.
Establishing the melting point of a diamond, scientists conducted experiments using a small natural mineral, the mass of which was 1/10 of a carat. Boiling of the surfaces of the material occurred under the influence of a shock wave created due to short-term laser pulses.
Boiling of the surfaces of the material occurred under the influence of a shock wave created due to short-term laser pulses.
The researchers managed to determine what indicator the melting point of diamond (in degrees) is only when pressure was created, which was 40 million times higher than the normal pressure of the atmosphere at sea level. With a decrease in pressure to 11 million atmospheres, solid particles began to form on the surface of the boiling mineral, which do not sink, but float like ice in water.
Where are diamonds found in the earth’s crust?
These minerals are extremely rare. However, industrial deposits are currently being developed on almost all continents of the globe. The only exception is Antarctica.
Until the middle of the 19th century, minerals were thought to form in river sediments. Later, the first diamond-bearing cavities were discovered in rocky mountain soil at a depth of several hundred meters.
According to scientists, some diamonds are between 100 million and 2. 5 billion years old. The researchers managed to get more “old” minerals of unearthly origin. The latter are brought to the planet along with meteorites, which were formed in outer space even before the formation of the solar system.
5 billion years old. The researchers managed to get more “old” minerals of unearthly origin. The latter are brought to the planet along with meteorites, which were formed in outer space even before the formation of the solar system.
Do molten diamonds exist in nature?
The melting point of diamond is so high that the mineral can no longer exist in boiling form on Earth. However, what about space objects? According to scientists, the melting point of diamond is maintained to this day in the depths of planets such as Neptune and Uranus. It is noteworthy that the latter are 10% formed from carbon, which is the structural basis of this mineral.
According to many scientists, on the above planets there are whole oceans of diamonds in liquid, boiling form. Such a hypothesis explains why the magnetic field of these celestial bodies behaves so strangely. After all, Neptune and Uranus are the only planets in the solar system whose geographic poles do not have a clear position and are literally spaced apart in space.

 Scientists have compared that this is how carbon behaves in the composition of the planets Neptune and Uranus, on the surface of these celestial bodies there are also oceans with liquid diamond. But in order to prove this assumption, it is necessary to send satellites to the planets, which at the moment cannot be quickly implemented.
Scientists have compared that this is how carbon behaves in the composition of the planets Neptune and Uranus, on the surface of these celestial bodies there are also oceans with liquid diamond. But in order to prove this assumption, it is necessary to send satellites to the planets, which at the moment cannot be quickly implemented. For this reason, no material is able to destroy or scratch its surface. He himself can damage any physical object.
For this reason, no material is able to destroy or scratch its surface. He himself can damage any physical object.