How do brine channels form in sea ice. What organisms live within these unique polar microhabitats. Why are brine pockets crucial for Arctic ecosystems. How do algae survive the dark polar winter in sea ice.
The Formation of Sea Ice and Brine Pockets
As seawater freezes in the frigid polar regions, a fascinating process unfolds. The freezing water forms freshwater ice crystals, leaving behind concentrated droplets of extra-salty liquid called brine. These brine droplets become trapped within small pockets and channels in the ice structure.
But how exactly do these brine pockets form? As temperatures plummet, the brine pockets shrink in size while simultaneously increasing in salinity. The high salt concentration prevents these pockets from freezing entirely, even in the depths of winter. This creates a network of microscopic habitats within the sea ice itself.
The Seasonal Cycle of Brine Channels
The brine channel system undergoes dramatic changes with the seasons:

- Winter: Brine pockets are small and highly concentrated
- Spring: Rising temperatures cause pockets to expand and merge
- Summer: Extensive networks of brine channels form throughout the ice
This dynamic environment provides crucial habitat for the microorganisms that become trapped in the ice during its formation. But what kinds of life can survive in such extreme conditions?
Life in the Ice: Microscopic Arctic Ecosystems
The brine channels in sea ice serve as home to a surprising diversity of microscopic life. During ice formation in autumn, various planktonic organisms become trapped within the developing ice structure. These include:
- Bacteria
- Photosynthetic algae
- Tiny worms
- Larvae of various species
Larger planktonic animals like copepods typically remain in the water below the ice, feeding on algae growing on the ice’s underside. But how do these trapped organisms survive the harsh Arctic winter?
Adaptations for Arctic Survival
Photosynthetic algae, a key component of this icy ecosystem, have developed remarkable adaptations. During the sunless winter months, they enter a dormant stage, dramatically lowering their energy requirements. This strategy allows them to survive until the return of sunlight in spring, at which point they “wake up” and resume growth.

The brine channels provide these organisms with:
- Protection from freezing
- A stable, if extreme, environment
- Access to nutrients from the water below
The Challenges of Life Under Ice
While the brine channels offer a refuge from freezing, life within sea ice comes with its own set of challenges. Organisms living in this environment must contend with:
- Extremely low temperatures
- High salinity
- Limited space
- Scarcity of light
The issue of light is particularly crucial for photosynthetic organisms like algae. Sunlight must penetrate through clouds, snow, and the ice itself before reaching the algae. Despite these dim conditions, ice algae have adapted to thrive in the lowest portions of the ice, where they can access nutrients from the water below.
The Ecological Importance of Sea Ice Ecosystems
The microscopic communities living within sea ice play a vital role in Arctic ecosystems. They serve as a critical food source for a variety of organisms, from tiny zooplankton to fish, and ultimately support larger predators like seals and polar bears.

Why are these ice-based ecosystems so important? They provide:
- A source of food during the winter months when other primary production is low
- An early spring bloom that jump-starts the Arctic food web
- Biodiversity in an otherwise harsh environment
As climate change continues to impact the Arctic, understanding these unique ecosystems becomes increasingly crucial. The loss of sea ice could have far-reaching consequences for the entire Arctic food web.
Studying Life in the Ice: Scientific Challenges and Innovations
Researching the microscopic life within sea ice presents unique challenges for scientists. How do researchers study organisms living in such an extreme and inaccessible environment?
Some key methods include:
- Ice core sampling
- Under-ice imaging and video
- Remote sensing technologies
- Laboratory simulations of brine channel environments
These techniques allow scientists to observe the structure of brine channels, measure environmental conditions, and study the organisms living within the ice. However, the delicate nature of these habitats means that any sampling must be done with great care to avoid disrupting the very ecosystems being studied.

Recent Discoveries and Ongoing Research
Scientific exploration of sea ice ecosystems continues to yield fascinating discoveries. Recent research has revealed:
- The presence of previously unknown microbial species adapted to extreme cold
- Complex food webs existing entirely within the ice
- The role of sea ice organisms in nutrient cycling and carbon sequestration
Ongoing studies are examining how these ecosystems might respond to climate change and the potential impacts on the broader Arctic environment.
The Impact of Climate Change on Sea Ice Ecosystems
As global temperatures rise, Arctic sea ice is undergoing dramatic changes. This shift has significant implications for the microscopic life that depends on sea ice habitats. How might climate change affect these unique ecosystems?
Potential impacts include:
- Reduction in overall sea ice extent and thickness
- Changes in the timing of ice formation and melt
- Alterations to brine channel structure and chemistry
- Disruption of the seasonal cycles that organisms depend on
These changes could have cascading effects throughout the Arctic food web. For example, if ice algae blooms occur earlier or are reduced in scale, it could impact the reproduction and survival of zooplankton, fish, and even marine mammals that rely on this seasonal pulse of productivity.

Adaptations and Resilience
While the challenges posed by climate change are significant, some research suggests that sea ice organisms may have some capacity to adapt. Studies have shown that:
- Some ice algae species can acclimate to changing light conditions
- Certain bacteria may be able to adjust to shifts in salinity and temperature
- The overall community composition may change, potentially maintaining ecosystem functions
However, the rate of current climate change may outpace the ability of many organisms to adapt, highlighting the urgent need for conservation efforts and climate mitigation strategies.
Brine Channels Beyond Earth: Implications for Astrobiology
The study of life in sea ice brine channels has implications that extend beyond our planet. These unique habitats serve as potential analogs for environments that might exist on other worlds in our solar system.
Why are astrobiologists interested in brine channels? They provide insights into:
- How life might survive in extremely cold environments
- The potential for life in subsurface oceans of icy moons like Europa or Enceladus
- Mechanisms for preserving biosignatures in ice
By studying the adaptations and survival strategies of organisms in Earth’s sea ice, scientists can better understand what to look for in the search for life beyond our planet.

Lessons for Extraterrestrial Exploration
Research on sea ice ecosystems is informing the development of technologies and strategies for exploring potentially habitable environments on other worlds. This includes:
- Design of instruments capable of detecting microbial life in ice
- Methods for sampling subsurface environments without contamination
- Understanding the chemical signatures that might indicate the presence of life
As we continue to explore our solar system, the lessons learned from Earth’s polar regions may prove invaluable in the search for life elsewhere in the universe.
Preserving Arctic Ecosystems: Conservation and Research Priorities
The unique and fragile nature of sea ice ecosystems underscores the importance of conservation efforts in the Arctic. As climate change continues to impact polar regions, what steps can be taken to protect these vital habitats?
Key priorities include:
- Reducing greenhouse gas emissions to slow the pace of Arctic warming
- Establishing protected areas to minimize human impacts on sea ice
- Supporting continued research to better understand sea ice ecosystems
- Developing sustainable practices for Arctic resource use and shipping
Conservation efforts must balance the needs of Arctic communities with the protection of these critical ecosystems. Collaborative approaches involving scientists, policymakers, and indigenous peoples are essential for developing effective strategies.

The Role of Public Awareness
Raising public awareness about the importance of sea ice ecosystems is crucial for garnering support for conservation efforts. Educational initiatives can help people understand:
- The intricate connections between sea ice and global climate
- The unique biodiversity found in Arctic environments
- The cultural significance of sea ice to Arctic indigenous communities
- How individual actions can contribute to Arctic conservation
By fostering a greater appreciation for these remote and often overlooked ecosystems, we can build a stronger foundation for their protection and preservation.
The study of brine channels and the life they contain offers a window into one of Earth’s most extreme and fascinating ecosystems. From the microscopic algae surviving the dark Arctic winter to the complex food webs that support iconic Arctic wildlife, sea ice plays a crucial role in the health of our planet. As we continue to unravel the mysteries of life in the ice, we gain not only a deeper understanding of our world but also valuable insights that may help us in the exploration of worlds beyond our own. The preservation of these unique habitats is not just a matter of scientific interest, but a critical step in maintaining the delicate balance of our global ecosystem.

Brine Channels | Ask A Biologist
show/hide words to know
Algae: eukaryotic organisms (ones that have membrane-enclosed cell parts) that live in fresh and salt water. They can be free floating or attached to a surface….more
Dissolve: to become part of a liquid.
Hibernate: the act of sleeping through the cold winter months, like some animals do to survive the winter… more
Ion: an atom or molecule that does not have the same number of electrons as it has protons. This gives the atom or molecule a negative or positive charge… more
The Start of Sea Ice
The molecular structure of water. Click on the image for more information.
Living in areas that freeze can make for dangerous mornings. You hold your arms out for balance as you walk out the door, taking each step carefully so you don’t slip on hidden ice. One way that people deal with icy walk ways is by pouring salt on the ice to make it melt. Salt isn’t warm, so how exactly is the salt affecting the ice?
Salt isn’t warm, so how exactly is the salt affecting the ice?
Salt does something special to the water in which it dissolves. It reduces the temperature at which the water freezes.
Early spring (top) and late spring (bottom) in the Arctic.
In the polar regions such as the Arctic, sea water does freeze in the winter, and then melts again in the late spring and summer. This is because the temperatures drops so much that even the loads of salt in the sea can’t prevent the water from freezing.
Even in the summer there is still ice in the polar regions because the temperatures remain very cold. The ice in the central regions of the Arctic around the North Pole is made up of several years’ worth of ice growth (we call that multi-year ice).
Bring Out Your Brine
Fresh and salt water cores compared. Can you guess which is which? Click on the image for more information.
When sea ice forms, freshwater ice is formed first, leaving behind droplets of salty liquid called brine.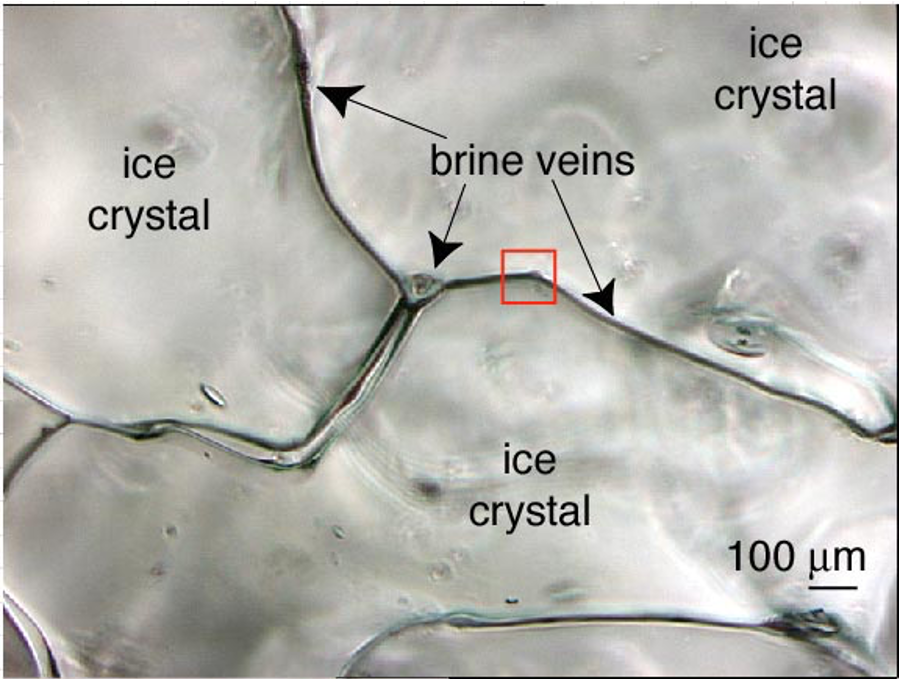 This brine can get trapped in pockets or channels in the ice. Microscopic organisms in the water get trapped in the brine.
This brine can get trapped in pockets or channels in the ice. Microscopic organisms in the water get trapped in the brine.
As the temperatures continue to decrease, the pockets of brine become smaller and smaller and the concentration of salt increases. These brine pockets never completely freeze because they have such a high concentration of salt.
In the spring when temperatures start to increase again, the exact opposite happens in these brine pockets. They begin to expand again because the ice around it melts and the pockets merge with other pockets and form long networks of channels in the ice. These channels are the living space (habitat) for the microscopic organisms that were trapped in the ice in the fall.
Winter (left) and spring (right) brine channels. Click on the image for more information.
Brine Channels Brim with Life
Whatever is in the water column during the fall might get trapped in the ice when the sea water forms. This includes any plankton (sea drifters), from bacteria to photosynthetic algae to worms and larvae.
Larger grazers such as the planktonic crustaceans (like copepods) will likely not be found inside the ice unless they swim into the brine channels in search of food. Mostly they will be under the ice in the water, picking algae from the bottom of the ice.
Color at the bottom ice core indicate algae living within the brine channels.
Photosynthetic algae are some of the plankton that become trapped in the ice during the fall. However, to make it through the winter with no light they enter a dormant or resting stage throughout the winter. This allows them to live but with low energy requirements (much like the polar bears hibernating above the ice). As soon as light becomes available for these organisms they “wake up” and start to grow again.
Life Under the Ice
The light from above must not only penetrate the clouds but then must pass through the snow and the ice to reach the organisms. This means the algae living in the ice have to deal with really dim light. But still they grow best in the lowest ice zone, where the ice meets the nutrient-rich open water. While this is the zone of the dimmest light, this is where they also get most of the nutrients needed for growth.
But still they grow best in the lowest ice zone, where the ice meets the nutrient-rich open water. While this is the zone of the dimmest light, this is where they also get most of the nutrients needed for growth.
To see what it’s like for the plankton under the ice, watch the video below.
This section of Ask A Biologist was funded by NSF Office of Polar Programs Grant Award number 1023140 as a part of Susanne Neuer’s research.
Read more about: Frozen Life
Brine channels, micro-habitat for ice algae
Science at Camp
admin Leave a comment
During the formation of sea ice, small spaces remain between the ice crystals and are filled with a salty liquid, known as brines.
These brines are trapped in pockets or channels in sea ice (Figure 1).
Figure 1: Sea ice structure with ice crystals and brine channels. Issued from Cryospheric Sciences.
When temperature decreases during winter, the size of brine channels/pockets reduces while the salinity of brine increases and prevents their freezing. During the formation of sea ice, numerous micro-organisms (bacteria, virus, larvae, worms and micro-algae) living in the water column were trapped and started to live in theses brine channels, rich in nutrients.
During spring, when the temperature starts to increase, the brine channels expand. With the increase of temperature, sea ice starts to melt and thereafter the brine pockets and the channels start to be inter-connected through the entire ice core (Figure 2).
Figure 2: Brine channels evolution from winter to spring. Issued from: ASU School of Life Sciences
At that time, ice algae are mostly concentrated at the bottom of sea ice to have access to the nutrient in the water column but they can also migrate through the channels in the ice if they need more sunlight (Figure 3).
Figure 3: Pictures of ice algae at the bottom of sea ice (left) and migration of ice algae in the ice core (right) as shown by the difference of color on the five first centimetres. Credit: Virginie Galindo
Meanwhile, larger grazers like copepods and amphipods come to the bottom of sea ice to graze some ice algae but they can also swim through the brine channels to find some food.
During Green Edge ice camp, the scientists measured the salinity and temperature on two complete ice cores every two days. With these parameters, they can estimate the brine volume and determine the periods of brine flushing associated with a decrease of salinity in the entire ice core. As shown on Figure 4, the salinity in the ice core decreased between 13 and 20 May, but mostly after the 13 June. The first decrease of salinity could be associated with a short drainage of brines, while the major decrease of salinity at the surface after 13 June is associated with the complete melt of the snow cover.
Figure 4. Time series of salinity profiles in the sea ice cores during the Green Edge ice camp 2016.
In fact, during the snow melt period, the melted snow penetrates the ice through the channels and flush most of the micro-organisms in the underlying water column. So the complete flushing of sea ice algae seems to be right now on the ice camp of Green Edge! This flushing of snow melt associated with the release of ice algae in the water column could seed the phytoplankton bloom underneath the ice. Should we observe the beginning of the phytoplankton bloom soon?
Virginie Galindo
Arctic OceanGreen EdgeGreen Edge TeamIce campPhytoplanktonPhytoplankton spring bloomSampling workScience at camptop
To understand the dynamics of the phytoplankton spring bloom and determine its role in the Arctic Ocean of tomorrow, including for human populations.
Salt lamps: what is the use?
Salt and salt lamps are different names for the same device.
Salt lamps are made from natural rock salt mined from the Himalayan mountains in Pakistan. The salt lamp looks like a ceiling carved from a natural mineral, equipped with a switch, a light bulb and a stand. The lamp works from the usual socket. Salt in a cold state has the ability to absorb moisture from the air, and when heated, it releases moisture.
Salt lamps are a natural air ionizer, a small salt spa at home. Using them indoors (apartment or office) is equivalent to being in a salt cave. Especially useful for those who are often sick.
BENEFIT AND HARM OF A SALT LAMP
The benefits of a salt lamp:
- cleans the air from dust and bacteria,
- maintains optimal humidity in the room,
- regular breathing with salt ions promotes the treatment of respiratory organs, allergies, skin diseases,
- provides prevention for asthma, dermatitis, diabetes, allergies, sinusitis, rheumatism, colds,
- relieves migraine headaches,
- strengthens the immune system and reduces vulnerability to colds and flu,
- improves sleep, mood and metabolism,
- neutralizes unpleasant odors in rooms
There are no contraindications to the use of a salt lamp, because it is made of natural materials. An exception can be considered individual intolerance, which is extremely rare.
An exception can be considered individual intolerance, which is extremely rare.
SELECTION CRITERIA FOR SALT LAMPS
- Salt lamp weight
The required weight of the salt lamp is taken from the calculation: 1 kg of salt per 3-4 square meters of the room. For example, a salt lamp of 3-5 kg is suitable for a room of 12 square meters. But you can always take several lamps of 1-2 kg at once.
- Form
Salt lamps come in many forms. You can choose any that you like best and suitable for your interior. The shape does not affect the quality of the product.
Natural Form The is the most popular type of salt lamp and is a piece of rock from which it was mined (they are usually referred to as “The Rock”). Most lamps do not go through additional cutting after being mined.
Artificial shape is a special shape in the form of a pyramid, ball or other shape.
HOW TO USE THE SALT LAMP
The salt lamp can be installed anywhere in the apartment or office space. The best place is where you spend the most time.
The best place is where you spend the most time.
Salt lamps are recommended to be installed:
- near appliances and household appliances (computers, TVs, etc.) to neutralize electromagnetic radiation,
- in the children’s room to strengthen the child’s immunity,
- in the bedroom for sound and healthy sleep,
- in the living room for relaxation and good rest of all family members
After purchase, unpack and keep the salt lamp unplugged for about 2 hours to dry naturally. At first, turn on the lamp for no more than 1 hour a day. After an adaptation period of 5-6 days, you can gradually increase the operating time up to 4-8 hours. It is allowed to turn on the device both during the day and at night.
It is not recommended to install the device near open windows and moisture sources such as decorative fountains and aquariums.
For safety reasons, the switched on lamp must not be covered.
WHERE TO BUY A SALT LAMP
Inspired by the benefits of a salt lamp? We invite you to the network of Salamat orthopedic salons, where a wide range of salt lamps is presented only from trusted suppliers.
IMPORTANT! Buy salt lamps only from specialized stores you trust. A real lamp is always made from only Himalayan pink salt and is never completely cheap. Counterfeits are useless and can harm your family’s health.
The range of salt lamps is presented in the catalog of the Salamat online store, follow this link.
Salt lamp is a beautiful and useful gift for your loved ones on any occasion and for any event.
With care for your family, the network of orthopedic salons “Salamat”.
Salt Riot — date, year, reason, essence, results
In 2023, exactly 375 years will have passed since one of the largest uprisings in the history of Russia — the Salt Riot. This rebellion, striking in its numbers, was a reflection of the social and economic problems of the 17th century. The boyars and the authorities felt the anger of the people, the rebellion came to many large cities. What caused the popular unrest and how the uprising ended – read in the Izvestia article.
Causes of the Salt Riot
The monetary policy of the state became the ground for discontent. In particular, food prices were strongly influenced by customs duties on the import of salt into Russia. At the same time, salt occupied an important place among the purchases of commoners, as it was the only available preservative.
In addition, the pockets of the peasants were greatly devastated by the tax reforms. The authorities returned previously canceled direct taxes and increased them for the “black settlements”, where the main population were small employees, merchants, artisans and other common people.
How the Salt Riot began
Dissatisfied with the innovations, the townspeople decided to petition Tsar Alexei Mikhailovich with a request to assemble the Zemsky Sobor and find justice for the boyars and corrupt officials. On June 1, the sovereign was returning from the Trinity-Sergius Monastery, when the crowd blocked his path and tried to pass a petition. However, the archers dispersed the peasants, 16 instigators were arrested.
However, the archers dispersed the peasants, 16 instigators were arrested.
Events of the Salt Riot
A desperate crowd began to riot. The commoners sacked the houses of the boyars, and the enraged people moved towards the Kremlin. The basis of the crowd were small merchants, artisans. Some dissatisfied nobles and even archers who sided with the people joined the procession.
Protesters set fire to entire neighborhoods and longed for reprisals against the boyars. The rebels brutally murdered Leonty Pleshcheev, the head of the Zemsky department, and executed the head of the Ambassadorial office, Nazariy Pure. The same fate soon befell the head of the Pushkar order, Peter Trakhaniotov.
Only Boris Morozov, the tsar’s favorite, escaped the massacre. The ruler himself promised to remove him from all affairs and exile him to the Kirillo-Belozersky monastery, which was done on the night of June 11-12.
Salt riot – the results of the uprising
The rebellion lasted about 10 days – by June 11, most of the centers of the uprising were liquidated.